Hepatitis D
| Hepatitis D | |
|---|---|
| Other names: Hepatitis delta | |
.jpg.webp) | |
| Jaundice | |
| Specialty | Gastroenterology, infectious disease |
| Symptoms | Fever, tiredness, nausea, vomiting, stomach ache, joint pains, jaundice[1][2] |
| Complications | Cirrhosis, liver failure, liver cancer[2] |
| Usual onset | 3-7 weeks after infection[1] |
| Types | Co-infection, superinfection[2] |
| Causes | Hepatitis D virus[2] |
| Risk factors | Hepatitis B[2] |
| Diagnostic method | Immunoglobulin G[3] |
| Prevention | Hepatitis B vaccine[2] |
| Treatment | Peginterferon alfa-2a[3] |
| Medication | Bulevirtide |
| Frequency | 5% of people chronic hepatitis B virus,[3] ~ 48-60 million globally[4] |
Hepatitis D is an infectious disease caused by the hepatitis delta virus (HDV) that affects the liver.[5] Symptoms tend to occur 3 to 7 weeks after becoming infected and include fever, tiredness, nausea, vomiting, stomach ache and joint pains.[1] The eyes become yellow, the urine may look dark and stool may appear a clay-color.[1] It occurs only in people with hepatitis B (HBV), either by being infected at the same time as HBV or by being an additional infection in a person already with HBV.[6] HDV and HBV infecting a person simultaneously is considered the most serious type of viral hepatitis due to its severity of complications.[3] These complications include a greater likelihood of experiencing liver failure in acute infections and a rapid progression to liver cirrhosis, with an increased risk of developing liver cancer in log-term infections.[7]
HDV is one of five known hepatitis viruses, the others being: A, B, C and E.[2] HDV is the only member of the only species in the genus Deltavirus, and requires the envelope of the Hepatitis B virus to become infectious.[8] The virus is transmitted through broken skin via injection or tattooing, or by contact with infected blood.[3]
Hepatitis D is considered in any person with features of hepatitis or is known to have hepatitis B surface antigen (HBsAg), who becomes suddenly unwell.[1] If available, diagnosis is confirmed by testing for antibodies against HDV and/or HDV RNA.[3] It may appear similar to the other types of hepatitis.[1]
Treatment with peginterferon alfa-2a may be an option, but does not always work well.[5] Hepatitis B vaccine offers protection to some.[2]
Globally, it affects around 5% of people with chronic hepatitis B virus.[3] It is more common in Eastern Europe, North Africa and the Brazilian rainforest.[5] In combination with hepatitis B virus, hepatitis D has the highest death rate of all the hepatitis infections, at 20%.[4] The exact number of infected people is not known, but estimated at 48 to 60 million globally as of 2020.[4]
Definition and types
Hepatitis D is a type of viral infection of the liver.[5] It occurs only in people with hepatitis B (HBV), either by being infected at the same time as HBV or by being an additional infection in a person already with HBV.[6]
Signs and symptoms
Presenting symptoms include fever, tiredness, nausea, vomiting, stomach ache, joint pains and jaundice.[1] The urine may look dark and stool may appear a clay-color.[1]
Complications
HDV and HBV infecting a person simultaneously is considered the most serious type of viral hepatitis due to its severity of complications.[3] These complications include a greater likelihood of experiencing liver failure in acute infections and a rapid progression to liver cirrhosis, with an increased risk of developing liver cancer in chronic infections.[7]
Virology
| Hepatitis delta virus | |
|---|---|
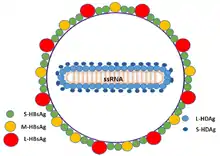 | |
| Schematic representation of the Hepatitis delta virus virion | |
| Virus classification | |
| (unranked): | Virus |
| Realm: | Ribozyviria |
| Family: | Kolmioviridae |
| Genus: | Deltavirus |
| Species[9] | |
| |
Structure and genome
The hepatitis delta viruses, or HDV, are eight species of negative-sense single-stranded RNA viruses (or virus-like particles) classified together as the genus Deltavirus, within the realm Ribozyviria.[10] The HDV virion is a small, spherical, enveloped particle with a 36 nm diameter; its viral envelope contains host phospholipids, as well as three proteins taken from the hepatitis B virus—the large, medium, and small hepatitis B surface antigens. This assembly surrounds an inner ribonucleoprotein (RNP) particle, which contains the genome surrounded by about 200 molecules of hepatitis D antigen (HDAg) for each genome. The central region of HDAg has been shown to bind RNA.[11] Several interactions are also mediated by a coiled-coil region at the N terminus of HDAg.[12][13]
The HDV genome is negative sense, single-stranded, closed circular RNA; with a genome of approximately 1700 nucleotides, HDV is the smallest "virus" known to infect animals. It has been proposed that HDV may have originated from a class of plant pathogens called viroids, which are much smaller than viruses.[14][15] Its genome is unique among animal viruses because of its high GC nucleotide content. Its nucleotide sequence is about 70% self-complementary, allowing the genome to form a partially double-stranded, rod-like RNA structure.[16] HDV strains are highly divergent; fusions of different strains exist and sequences had been deposited in public databases employing different start sites for the circular viral DNA involved. This had resulted in something of chaos with respect to molecular classification of this virus, a situation which has been resolved recently with the adoption of a proposed reference genome and a uniform classification system.[17]
Life cycle
Like hepatitis B, HDV gains entry into liver cells via the NTCP[18] bile transporter. HDV recognizes its receptor via the N-terminal domain of the large hepatitis B surface antigen, HBsAg.[19] Mapping by mutagenesis of this domain has shown that amino acid residues 9–15 make up the receptor-binding site.[20] After entering the hepatocyte, the virus is uncoated and the nucleocapsid translocated to the nucleus due to a signal in HDAg[21] Since the HDV genome does not code for an RNA polymerase to replicate the virus’ genome, the virus makes use of the host cellular RNA polymerases. Initially thought to use just RNA polymerase II,[22][23] now RNA polymerases I and III have also been shown to be involved in HDV replication.[24] Normally RNA polymerase II utilizes DNA as a template and produces mRNA. [25][26]
The RNA polymerases treat the RNA genome as double-stranded DNA due to the folded rod-like structure it is in. Three forms of RNA are made; circular genomic RNA, circular complementary antigenomic RNA, and a linear polyadenylated antigenomic RNA, which is the mRNA containing the open reading frame for the HDAg. Synthesis of antigenomic RNA occurs in the nucleolus, mediated by RNA polymerase I, whereas synthesis of genomic RNA takes place in the nucleoplasm, mediated by RNA polymerase II.[27] HDV RNA is synthesized first as linear RNA that contains many copies of the genome. The genomic and antigenomic RNA contain a sequence of 85 nucleotides, the hepatitis delta virus ribozyme, that acts as a ribozyme, which self-cleaves the linear RNA into monomers. These monomers are then ligated to form circular RNA.[28][29]
Delta antigens
| Hepatitis delta virus delta antigen | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 oligomerization domain of hepatitis delta antigen | |||||||||
| Identifiers | |||||||||
| Symbol | HDV_ag | ||||||||
| Pfam | PF01517 | ||||||||
| InterPro | IPR002506 | ||||||||
| SCOP2 | 1a92 / SCOPe / SUPFAM | ||||||||
| |||||||||
A significant difference between viroids and HDV is that, while viroids produce no proteins, HDV is known to produce one protein, namely HDAg. It comes in two forms; a 27kDa large-HDAg, and a small-HDAg of 24kDa. The N-terminals of the two forms are identical, they differ by 19 more amino acids in the C-terminal of the large HDAg.[30] Both isoforms are produced from the same reading frame which contains an UAG stop codon at codon 196, which normally produces only the small-HDAg. However, editing by cellular enzyme adenosine deaminase-1 changes the stop codon to UGG, allowing the large-HDAg to be produced.[30][31] Despite having 90% identical sequences, these two proteins play diverging roles during the course of an infection. HDAg-S is produced in the early stages of an infection and enters the nucleus and supports viral replication. HDAg-L, in contrast, is produced during the later stages of an infection, acts as an inhibitor of viral replication, and is required for assembly of viral particles.[32][33][34]
Antigenic loop infectivity
The HDV envelope protein has three of the HBV surface proteins anchored to it. The S region of the genome is most commonly expressed and its main function is to assemble subviral particles. HDV antigen proteins combine with the viral genome to form a ribonucleoprotein (RNP) which when enveloped with the subviral particles can form viral-like particles that are almost identical to mature HDV, but they are not infectious. Researchers had concluded that the determinant of infectivity of HDV was within the N-terminal pre-S1 domain of the large protein (L). It was found to be a mediator in binding to the cellular receptor. Researchers Georges Abou Jaoudé and Camille Sureau published an article in 2005 that studied the role of the antigenic loop, found in HDV envelope proteins, in the infectivity of the virus. The antigenic loop, like the N-terminal pre-S1 domain of the large protein, is exposed at the virion surface. Jaoudé and Sureau's study provided evidence that the antigenic loop may be an important factor in HDV entry into the host cell and by mutating parts of the antigenic loop, the infectivity of HDV may be minimized.[35]
Transmission
The routes of transmission of hepatitis D are similar to those for hepatitis B. Infection is largely restricted to persons at high risk of hepatitis B infection, particularly injecting drug users and persons receiving clotting factor concentrates. Worldwide more than 15 million people are co-infected. HDV is rare in most developed countries, and is mostly associated with intravenous drug use. However, HDV is much more common in the immediate Mediterranean region, sub-Saharan Africa, the Middle East, and the northern part of South America.[36] In all, about 20 million people may be infected with HDV.[37]
Diagnosis
Hepatitis D is considered in any person with features of hepatitis or is known to have hepatitis B surface antigen (HBsAg), who becomes suddenly unwell.[1] Diagnosis is confirmed by testing for antibodies against HDV and/or HDV RNA.[1] It may appear similar to the other types of hepatitis.[1]
Prevention
The vaccine for hepatitis B protects against hepatitis D virus because of the latter's dependence on the presence of hepatitis B virus for it to replicate.[38][39] In absence of a specific vaccine against delta virus, the vaccine against HBV must be given soon after birth in risk groups.[3]
Treatment

Hepatitis D is generally considered the dominant virus over hepatitis B except in rare instances. Current established treatments for chronic hepatitis D include conventional or pegylated interferon alpha therapy.[40]
Latest evidence suggests that pegylated interferon alpha is effective in reducing the viral load and the effect of the disease during the time the drug is given, but the benefit generally stops if the drug is discontinued.[41]
The efficiency of this treatment does not usually exceed ~20%, and late relapse after therapy has been reported.[42]
In May 2020, the Committee for Medicinal Products for Human Use of the European Medicines Agency approved the antiviral Hepcludex (bulevirtide) to treat hepatitis D and B. Bulevirtide binds and inactivates the sodium/bile acid cotransporter, blocking both viruses from entering hepatocytes.[43][44]
Epidemiology

Those afflicted are individuals who have been infected with Hepatitis B virus as the Hepatitis D (HDV) virus needs the HBsAg (hepatitis B surface antigen) for packaging and transmission. The disease is present worldwide. Infection with HDV is a major medical scourge in low income regions of the globe in which the HBV remains endemic.[46] It is therefore most prevalent in countries where HBV infection is also common, currently the Amazon basin and low income regions of Asia and Africa. Improved measures to control HBV in industrialised countries (such as by vaccination) have also reduced the prevalence of HDV, with the main remaining at-risk populations in those countries being injection drug users and immigrants from endemic HDV areas.[46]
History
Hepatitis D virus was first reported in 1977 as a nuclear antigen in patients infected with HBV who had severe liver disease.[47] This nuclear antigen was then thought to be a hepatitis B antigen and was called the delta antigen. Subsequent experiments in chimpanzees showed that the hepatitis delta antigen (HDAg) was a structural part of a pathogen that required HBV infection to produce a complete viral particle.[48] The entire genome was cloned and sequenced in 1986. It was subsequently placed in its own genus: Deltavirus.[49][50]
Evolution
Three genotypes (I–III) were originally described. Genotype I has been isolated in Europe, North America, Africa and some Asia. Genotype II has been found in Japan, Taiwan, and Yakutia (Russia). Genotype III has been found exclusively in South America (Peru, Colombia, and Venezuela). Some genomes from Taiwan and the Okinawa islands have been difficult to type but have been placed in genotype 2. However it is now known that there are at least 8 genotypes of this virus (HDV-1 to HDV-8).[51] Phylogenetic studies suggest an African origin for this pathogen.[36]
An analysis of 36 strains of genotype 3 estimated that the most recent common ancestor of these strains originated around 1930.[52] This genotype spread exponentially from early 1950s to the 1970s in South America. The substitution rate was estimated to be 1.07×10−3 substitutions per site per year. Another study[53] found an overall evolution rate of 3.18×10−3 substitutions per site per year. The mutation rate varied with position : the hypervariable region evolved faster (4.55×10−3 substitutions per site per year) than the hepatitis delta antigen coding region (2.60×10−3 substitutions per site per year) and the autocatalytic region (1.11×10−3 substitutions per site per year). A third study suggested a mutation rate between 9.5×10−3 to 1.2×10−3 substitutions/site/year.[54]
Genotypes, with the exception of type 1, appear to be restricted to certain geographical areas: HDV-2 (previously HDV-IIa) is found in Japan, Taiwan and Yakutia; HDV-4 (previously HDV-IIb) in Japan and Taiwan; HDV-3 in the Amazonian region; HDV-5, HDV-6, HDV-7 and HDV-8 in Africa.[55] Genotype 8 has also been isolated from South America. This genotype is usually only found in Africa and may have been imported into South America during the slave trade.[56]
HDV-specific CD8+ T cells can control the virus, but it has been found HDV mutates to escape detection by CD8+ T cells.[57]
Related species
A few other viruses with similarity to HDV have been described in species other than humans. Unlike HDV, none of them depend on a Hepadnaviridae (HBV family) virus to replicate. These agents have rod-like structure, a delta antigen, and a ribozyme.[58] HDV and all such relatives are classified in their own realm, Ribozyviria, by the International Committee on Taxonomy of Viruses.[59]
References
- 1 2 3 4 5 6 7 8 9 10 11 "What is Hepatitis D - FAQ | CDC". www.cdc.gov. 3 December 2020. Archived from the original on 5 November 2021. Retrieved 5 December 2021.
- 1 2 3 4 5 6 7 8 "Hepatitis D | NIDDK". National Institute of Diabetes and Digestive and Kidney Diseases. Archived from the original on 9 October 2019. Retrieved 10 September 2019.
- 1 2 3 4 5 6 7 8 9 "Hepatitis D". www.who.int. Archived from the original on 30 April 2018. Retrieved 10 September 2019.
- 1 2 3 Miao Z, Zhang S, Ou X, Li S, Ma Z, Wang W, Peppelenbosch MP, Liu J, Pan Q (April 2020). "Estimating the Global Prevalence, Disease Progression, and Clinical Outcome of Hepatitis Delta Virus Infection". The Journal of Infectious Diseases. 221 (10): 1677–1687. doi:10.1093/infdis/jiz633. PMC 7184909. PMID 31778167.
- 1 2 3 4 Foster, Graham; O'Brien, Alastair (2020). "34. Liver disease". In Feather, Adam; Randall, David; Waterhouse, Mona (eds.). Kumar and Clark's Clinical Medicine (10th ed.). Elsevier. p. 1282. ISBN 978-0-7020-7870-5. Archived from the original on 2021-12-11. Retrieved 2021-12-07.
- 1 2 "Hepatitis (Viral) NIDDK". The National Institute of Diabetes and Digestive and Kidney Diseases. Archived from the original on 2016-12-29. Retrieved 2020-06-19.
- 1 2 Fattovich G, Giustina G, Christensen E, Pantalena M, Zagni I, Realdi G, Schalm SW (March 2000). "Influence of hepatitis delta virus infection on morbidity and mortality in compensated cirrhosis type B. The European Concerted Action on Viral Hepatitis (Eurohep)". Gut. 46 (3): 420–6. doi:10.1136/gut.46.3.420. PMC 1727859. PMID 10673308.
- ↑ Magnius L, Taylor J, Mason WS, Sureau C, Dény P, Norder H (December 2018). "ICTV Virus Taxonomy Profile: Deltavirus". The Journal of General Virology. 99 (12): 1565–1566. doi:10.1099/jgv.0.001150. PMID 30311870.
- ↑ "ICTV 9th Report (2011) Deltavirus". International Committee on Taxonomy of Viruses (ICTV). Archived from the original on 30 January 2019. Retrieved 30 January 2019.
- ↑ "Virus Taxonomy: 2020 Release". International Committee on Taxonomy of Viruses (ICTV). March 2021. Archived from the original on 20 March 2020. Retrieved 5 August 2021.
- ↑ Poisson F, Roingeard P, Baillou A, Dubois F, Bonelli F, Calogero RA, Goudeau A (November 1993). "Characterization of RNA-binding domains of hepatitis delta antigen". The Journal of General Virology. 74 (Pt 11): 2473–8. doi:10.1099/0022-1317-74-11-2473. PMID 8245865.
- ↑ Zuccola HJ, Rozzelle JE, Lemon SM, Erickson BW, Hogle JM (July 1998). "Structural basis of the oligomerization of hepatitis delta antigen". Structure. 6 (7): 821–30. doi:10.1016/S0969-2126(98)00084-7. PMID 9687364.
- ↑ This article incorporates text from the public domain Pfam and InterPro: IPR002506
- ↑ Elena SF, Dopazo J, Flores R, Diener TO, Moya A (July 1991). "Phylogeny of viroids, viroidlike satellite RNAs, and the viroidlike domain of hepatitis delta virus RNA". Proceedings of the National Academy of Sciences of the United States of America. 88 (13): 5631–4. Bibcode:1991PNAS...88.5631E. doi:10.1073/pnas.88.13.5631. PMC 51931. PMID 1712103.
- ↑ Sureau C (2006). "The role of the HBV envelope proteins in the HDV replication cycle". Hepatitis Delta Virus. Current Topics in Microbiology and Immunology. Vol. 307. pp. 113–31. doi:10.1007/3-540-29802-9_6. ISBN 978-3-540-29801-4. PMID 16903223.
- ↑ Saldanha JA, Thomas HC, Monjardino JP (July 1990). "Cloning and sequencing of RNA of hepatitis delta virus isolated from human serum". The Journal of General Virology. 71 (7): 1603–6. doi:10.1099/0022-1317-71-7-1603. PMID 2374010.
- ↑ Miao Z, Zhang S, Ma Z, Hakim MS, Wang W, Peppelenbosch MP, Pan Q (January 2019). "Recombinant identification, molecular classification and proposed reference genomes for hepatitis delta virus". Journal of Viral Hepatitis. 26 (1): 183–190. doi:10.1111/jvh.13010. PMC 7379554. PMID 30260538.
- ↑ Yan H, Zhong G, Xu G, He W, Jing Z, Gao Z, Huang Y, Qi Y, Peng B, Wang H, Fu L, Song M, Chen P, Gao W, Ren B, Sun Y, Cai T, Feng X, Sui J, Li W (November 2012). "Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus". eLife. 1: e00049. doi:10.7554/eLife.00049. PMC 3485615. PMID 23150796.
- ↑ Engelke M, Mills K, Seitz S, Simon P, Gripon P, Schnölzer M, Urban S (April 2006). "Characterization of a hepatitis B and hepatitis delta virus receptor binding site". Hepatology. 43 (4): 750–60. doi:10.1002/hep.21112. PMID 16557545. S2CID 23549907.
- ↑ Schulze A, Schieck A, Ni Y, Mier W, Urban S (February 2010). "Fine mapping of pre-S sequence requirements for hepatitis B virus large envelope protein-mediated receptor interaction". Journal of Virology. 84 (4): 1989–2000. doi:10.1128/JVI.01902-09. PMC 2812397. PMID 20007265.
- ↑ Xia YP, Yeh CT, Ou JH, Lai MM (February 1992). "Characterization of nuclear targeting signal of hepatitis delta antigen: nuclear transport as a protein complex". Journal of Virology. 66 (2): 914–21. doi:10.1128/JVI.66.2.914-921.1992. PMC 240792. PMID 1731113.
- ↑ Lehmann E, Brueckner F, Cramer P (November 2007). "Molecular basis of RNA-dependent RNA polymerase II activity". Nature. 450 (7168): 445–9. Bibcode:2007Natur.450..445L. doi:10.1038/nature06290. hdl:11858/00-001M-0000-0015-7EE1-9. PMID 18004386. S2CID 4393153.
- ↑ Filipovska J, Konarska MM (January 2000). "Specific HDV RNA-templated transcription by pol II in vitro". RNA. 6 (1): 41–54. doi:10.1017/S1355838200991167. PMC 1369892. PMID 10668797.
- ↑ Greco-Stewart VS, Schissel E, Pelchat M (March 2009). "The hepatitis delta virus RNA genome interacts with the human RNA polymerases I and III". Virology. 386 (1): 12–5. doi:10.1016/j.virol.2009.02.007. PMID 19246067.
- ↑ Abbas, Zaigham; Afzal, Rafia (27 December 2013). "Life cycle and pathogenesis of hepatitis D virus: A review". World Journal of Hepatology. 5 (12): 666–675. doi:10.4254/wjh.v5.i12.666. ISSN 1948-5182. Archived from the original on 11 December 2021. Retrieved 6 December 2021.
- ↑ Mentha, Nathalie; Clément, Sophie; Negro, Francesco; Alfaiate, Dulce (29 March 2019). "A review on hepatitis D: From virology to new therapies". Journal of Advanced Research. 17: 3–15. doi:10.1016/j.jare.2019.03.009. ISSN 2090-1232. Archived from the original on 11 December 2021. Retrieved 10 December 2021.
- ↑ Li YJ, Macnaughton T, Gao L, Lai MM (July 2006). "RNA-templated replication of hepatitis delta virus: genomic and antigenomic RNAs associate with different nuclear bodies". Journal of Virology. 80 (13): 6478–86. doi:10.1128/JVI.02650-05. PMC 1488965. PMID 16775335.
- ↑ Branch AD, Benenfeld BJ, Baroudy BM, Wells FV, Gerin JL, Robertson HD (February 1989). "An ultraviolet-sensitive RNA structural element in a viroid-like domain of the hepatitis delta virus". Science. 243 (4891): 649–52. Bibcode:1989Sci...243..649B. doi:10.1126/science.2492676. PMID 2492676.
- ↑ Wu HN, Lin YJ, Lin FP, Makino S, Chang MF, Lai MM (March 1989). "Human hepatitis delta virus RNA subfragments contain an autocleavage activity". Proceedings of the National Academy of Sciences of the United States of America. 86 (6): 1831–5. Bibcode:1989PNAS...86.1831W. doi:10.1073/pnas.86.6.1831. PMC 286798. PMID 2648383.
- 1 2 Weiner AJ, Choo QL, Wang KS, Govindarajan S, Redeker AG, Gerin JL, Houghton M (February 1988). "A single antigenomic open reading frame of the hepatitis delta virus encodes the epitope(s) of both hepatitis delta antigen polypeptides p24 delta and p27 delta". Journal of Virology. 62 (2): 594–9. doi:10.1128/JVI.62.2.594-599.1988. PMC 250573. PMID 2447291.
- ↑ Jayan GC, Casey JL (December 2002). "Inhibition of hepatitis delta virus RNA editing by short inhibitory RNA-mediated knockdown of ADAR1 but not ADAR2 expression". Journal of Virology. 76 (23): 12399–404. doi:10.1128/JVI.76.23.12399-12404.2002. PMC 136899. PMID 12414985.
- ↑ Sato S, Cornillez-Ty C, Lazinski DW (August 2004). "By inhibiting replication, the large hepatitis delta antigen can indirectly regulate amber/W editing and its own expression". Journal of Virology. 78 (15): 8120–34. doi:10.1128/JVI.78.15.8120-8134.2004. PMC 446097. PMID 15254184.
- ↑ Taylor JM (2006). "Structure and replication of hepatitis delta virus RNA". Hepatitis Delta Virus. Current Topics in Microbiology and Immunology. Vol. 307. pp. 1–23. doi:10.1007/3-540-29802-9_1. ISBN 978-3-540-29801-4. PMID 16903218.
- ↑ Chang MF, Chen CJ, Chang SC (February 1994). "Mutational analysis of delta antigen: effect on assembly and replication of hepatitis delta virus". Journal of Virology. 68 (2): 646–53. doi:10.1128/JVI.68.2.646-653.1994. PMC 236498. PMID 8289368.
- ↑ Jaoudé GA, Sureau C (August 2005). "Role of the antigenic loop of the hepatitis B virus envelope proteins in infectivity of hepatitis delta virus". Journal of Virology. 79 (16): 10460–6. CiteSeerX 10.1.1.570.4147. doi:10.1128/jvi.79.16.10460-10466.2005. PMC 1182656. PMID 16051838.
- 1 2 Radjef N, Gordien E, Ivaniushina V, Gault E, Anaïs P, Drugan T, Trinchet JC, Roulot D, Tamby M, Milinkovitch MC, Dény P (March 2004). "Molecular phylogenetic analyses indicate a wide and ancient radiation of African hepatitis delta virus, suggesting a deltavirus genus of at least seven major clades". Journal of Virology. 78 (5): 2537–44. doi:10.1128/JVI.78.5.2537-2544.2004. PMC 369207. PMID 14963156.
- ↑ Taylor JM (January 2006). "Hepatitis delta virus". Virology. 344 (1): 71–6. doi:10.1016/j.virol.2005.09.033. PMID 16364738.
- ↑ "U.S. National Library of Medicine "Delta Agent (hepatitis D)"". Archived from the original on 2014-01-02. Retrieved 2021-10-22.
- ↑ Tayor JM (2009). Desk Encyclopedia of Human and Medical Virology. Boston: Academic Press. p. 121. ISBN 978-0-12-375147-8.
- ↑ Yurdaydin C, Idilman R (August 2015). "Therapy of Delta Hepatitis". Cold Spring Harbor Perspectives in Medicine. 5 (10): a021543. doi:10.1101/cshperspect.a021543. PMC 4588130. PMID 26253093.
- ↑ Abbas Z, Khan MA, Salih M, Jafri W (December 2011). Abbas Z (ed.). "Interferon alpha for chronic hepatitis D". The Cochrane Database of Systematic Reviews (12): CD006002. doi:10.1002/14651858.CD006002.pub2. PMC 6823236. PMID 22161394. Archived from the original on 2020-07-29. Retrieved 2021-10-22.
- ↑ Pascarella S, Negro F (January 2011). "Hepatitis D virus: an update". Liver International. 31 (1): 7–21. doi:10.1111/j.1478-3231.2010.02320.x. PMID 20880077. S2CID 29142477.
- ↑ Francisco EM (2020-05-29). "Hepcludex". European Medicines Agency. Archived from the original on 2020-06-15. Retrieved 2020-08-06.
- ↑ "Bulevirtide - MYR Pharma - AdisInsight". adisinsight.springer.com. Archived from the original on 2017-08-15. Retrieved 2020-08-06.
MYR Pharmaceuticals receives Conditional Marketing Authorisation by the European Commission for bulevirtide in the European Union for Hepatitis B and D
- ↑ Rizzetto M (2020). "Epidemiology of the Hepatitis D virus". WikiJournal of Medicine. 7: 7. doi:10.15347/wjm/2020.001. Archived from the original on 2021-10-19. Retrieved 2021-10-22.
- 1 2 Rizzetto M (July 2015). "Hepatitis D Virus: Introduction and Epidemiology". Cold Spring Harbor Perspectives in Medicine. 5 (7): a021576. doi:10.1101/cshperspect.a021576. PMC 4484953. PMID 26134842.
- ↑ Rizzetto M, Canese MG, Aricò S, Crivelli O, Trepo C, Bonino F, Verme G (December 1977). "Immunofluorescence detection of new antigen-antibody system (delta/anti-delta) associated to hepatitis B virus in liver and in serum of HBsAg carriers". Gut. 18 (12): 997–1003. doi:10.1136/gut.18.12.997. PMC 1411847. PMID 75123.
- ↑ Rizzetto M, Canese MG, Purcell RH, London WT, Sly LD, Gerin JL (Nov–Dec 1981). "Experimental HBV and delta infections of chimpanzees: occurrence and significance of intrahepatic immune complexes of HBcAg and delta antigen". Hepatology. 1 (6): 567–74. doi:10.1002/hep.1840010602. PMID 7030907. S2CID 83892580.
- ↑ Wang KS, Choo QL, Weiner AJ, Ou JH, Najarian RC, Thayer RM, Mullenbach GT, Denniston KJ, Gerin JL, Houghton M (Oct 9–15, 1986). "Structure, sequence and expression of the hepatitis delta (delta) viral genome". Nature. 323 (6088): 508–14. Bibcode:1986Natur.323..508W. doi:10.1038/323508a0. PMID 3762705. S2CID 4265339.
- ↑ Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA (2005). "Deltavirus". Eight Report of the International Committee on Taxonomy of Viruses. London: 735–8.
- ↑ Celik I, Karatayli E, Cevik E, Kabakçi SG, Karatayli SC, Dinç B, et al. (December 2011). "Complete genome sequences and phylogenetic analysis of hepatitis delta viruses isolated from nine Turkish patients". Archives of Virology. 156 (12): 2215–20. doi:10.1007/s00705-011-1120-y. PMID 21984217. S2CID 31356.
- ↑ Alvarado-Mora MV, Romano CM, Gomes-Gouvêa MS, Gutierrez MF, Carrilho FJ, Pinho JR (August 2011). "Dynamics of hepatitis D (delta) virus genotype 3 in the Amazon region of South America". Infection, Genetics and Evolution. 11 (6): 1462–8. doi:10.1016/j.meegid.2011.05.020. PMID 21645647.
- ↑ Chao YC, Tang HS, Hsu CT (August 1994). "Evolution rate of hepatitis delta virus RNA isolated in Taiwan". Journal of Medical Virology. 43 (4): 397–403. doi:10.1002/jmv.1890430414. PMID 7964650. S2CID 22539505.
- ↑ Homs M, Rodriguez-Frias F, Gregori J, Ruiz A, Reimundo P, Casillas R, Tabernero D, Godoy C, Barakat S, Quer J, Riveiro-Barciela M, Roggendorf M, Esteban R, Buti M (2016). "Evidence of an Exponential Decay Pattern of the Hepatitis Delta Virus Evolution Rate and Fluctuations in Quasispecies Complexity in Long-Term Studies of Chronic Delta Infection". PLOS ONE. 11 (6): e0158557. Bibcode:2016PLoSO..1158557H. doi:10.1371/journal.pone.0158557. PMC 4928832. PMID 27362848.
- ↑ Le Gal F, Gault E, Ripault MP, Serpaggi J, Trinchet JC, Gordien E, Dény P (September 2006). "Eighth major clade for hepatitis delta virus". Emerging Infectious Diseases. 12 (9): 1447–50. doi:10.3201/eid1209.060112. PMC 3294742. PMID 17073101.
- ↑ Barros LM, Gomes-Gouvêa MS, Pinho JR, Alvarado-Mora MV, Dos Santos A, Mendes-Corrêa MC, Caldas AJ, Sousa MT, Santos MD, Ferreira AS (September 2011). "Hepatitis Delta virus genotype 8 infection in Northeast Brazil: inheritance from African slaves?". Virus Research. 160 (1–2): 333–9. doi:10.1016/j.virusres.2011.07.006. PMID 21798297.
- ↑ Karimzadeh H, Kiraithe MM, Oberhardt V, Salimi Alizei E, Bockmann J, Schulze Zur Wiesch J, et al. (May 2019). "Mutations in Hepatitis D Virus Allow It to Escape Detection by CD8+ T Cells and Evolve at the Population Level". Gastroenterology. 156 (6): 1820–1833. doi:10.1053/j.gastro.2019.02.003. PMC 6486497. PMID 30768983.
- ↑ Paraskevopoulou S, Pirzer F, Goldmann N, Schmid J, Corman VM, Gottula LT, et al. (July 2020). "Mammalian deltavirus without hepadnavirus coinfection in the neotropical rodent Proechimys semispinosus". Proceedings of the National Academy of Sciences of the United States of America. 117 (30): 17977–17983. doi:10.1073/pnas.2006750117. PMC 7395443. PMID 32651267.
- ↑ Hepojoki J, Hetzel U, Paraskevopoulou S, Drosten C, Harrach B, Zerbini M, Koonin EV, Krupovic M, Dolja V, Kuhn JH (6 December 2020). "Create one new realm (Ribozyviria) including one new family (Kolmioviridae) including genus Deltavirus and seven new genera for a total of 15 species" (docx). International Committee on Taxonomy of Viruses (ICTV). Archived from the original on 16 May 2021. Retrieved 27 May 2021.
Bibliography
- Specter SC, ed. (1999). Viral Hepatitis: Diagnosis, Therapy, and Prevention. Humana Press. ISBN 0-89603-424-0.
- da Fonseca JC (2004). "[Hepatitis fulminant in Brazilian Amazon]". Revista da Sociedade Brasileira de Medicina Tropical. 37. 37 Suppl 2: 93–5. doi:10.1590/s0037-86822004000700015. PMID 15586904.
- Bensabath G, Soares M (2004). "[The evolution of knowledge about viral hepatitis in Amazon region: from epidemiology and etiology to the prophilaxy]". Revista da Sociedade Brasileira de Medicina Tropical. 37 () Suppl 2): 14–26. doi:10.1590/S0037-86822004000700003. PMID 15586892.
- Fonseca JC, Souza RA, Brasil LM, Araújo JR, Ferreira LC (2004). "Fulminant hepatic failure in children and adolescents in Northern Brazil". Revista da Sociedade Brasileira de Medicina Tropical. 37 (1): 67–9. doi:10.1590/S0037-86822004000100019. PMID 15042190.
External links
- World Health Organization Fact sheet on Hepatitis D
- ICTV Report: Deltavirus Archived 2021-12-11 at the Wayback Machine
- Viralzone: Deltavirus Archived 2010-06-13 at the Wayback Machine
- "Hepatitis delta virus". NCBI Taxonomy Browser. 12475. Archived from the original on 2021-11-09. Retrieved 2021-10-22.
| Classification |
|---|