Nepetalactone
 (cis,trans)-nepetalactone | |
 (cis,trans)-nepetalactone | |
| Names | |
|---|---|
| IUPAC name
4,7-Dimethyl-5,6,7,7a-tetrahydrocyclopenta[c]pyran-1(4aH)-one | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C10H14O2 |
| Molar mass | 166.220 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Nepetalactone is a name for multiple iridoid analog stereoisomers. Nepetalactones are produced by Nepeta cataria (catnip) and many other plants belonging to the genus Nepeta, in which they protect these plants from herbivorous insects by functioning as insect repellents. They are also produced by many aphids, in which they are sex pheromones.[1] Nepetalactones are cat attractants, and cause the behavioral effects that catnip induces in domestic cats. However, they affect visibly only about 2/3 of adult cats. They produce similar behavioral effects in many other Felidae, especially in lions and jaguars.[2] In 1941, the research group of Samuel M. McElvain was the first to determine the structures of nepetalactones and several related compounds.[3][4]
Structure and properties
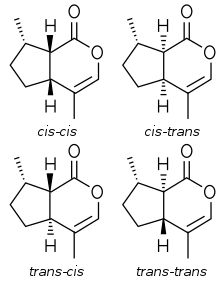
Nepetalactone has 3 chiral centers, two at the fusion of the two rings, and one where the methyl group attaches to the cyclopentane ring. Thus, it has 8 (=23) stereoisomers. The terms cis and trans are used to refer to the relative stereochemistry at the ring fusion, and also to the methyl group as compared to the lactone on the cyclopentane.
(cis,trans)-nepetalactone is a colorless oil.[5] Its boiling point is 71°C at 0.05 mmHg. At 25 °C, its density is 1.0663 g/mL and refractive index 1.4859.[6]
Natural occurrence
Plants belonging to the Nepeta genus produce 4 different nepetalactone stereoisomers: (cis,cis)-, (cis,trans)-, (trans,cis)- and (trans,trans)-nepetalactone. Their relative occurrence varies among plant species.[7] Small amounts of (cis,trans)- and (trans,cis)-nepetalactone also occur in the wood of Lonicera tatarica, but its cat attractant effects are assumed to be caused by actinidine, which occurs in it in higher concentrations.[8]
Nepetalactones are also produced by many aphids, in which they function as sex pheromones. The most common isomer in aphids is (cis,trans)-nepetalactone. Aphids also commonly produce a structurally related (1R,4αS,7S,7αR)-nepetalactol, which is also an aphid sex pheromone. Relative concentrations of these two compounds varies among aphid species.[9]
Effects in felines
Duration and efficacy variation
Nepetalactones affect domestic cats via nasal mucosa. Oral ingestion has no effects.[5] They induce noticeable behavioral effects in about 2/3 of adult cats. However, all cats are probably affected by them, but the effects in 1/3 of adult cats are less visible. Nepetalactones do not noticeably affect kittens that are less than 3 months old. Their effects also tend to be less pronounced in neutered cats in comparison to non-neutered cats, but not significantly.[2]
The effects of nepetalactones begin quickly in domestic cats, and last for 5–15 minutes. Cats develop drug tolerance towards nepetalactones after exposure. The tolerance lasts for a few hours.[5]
Lions (Panthera leo) and jaguars (Panthera onca) are sensitive to nepetalactones. Their effects can last in them for up to 60 minutes.[2][10] They also affect leopards (Panthera pardus). They do not affect tigers (Panthera tigris), bobcats (Lynx rufus), cougars (Puma concolor)[10] or oncillas (Leopardus tigrinus).[11]
Effects
2/3 of adult domestic cats begin to lick, sniff, eat, scratch or roll over the nepetalactone source after being exposed to it. They may also begin pawing, shaking their heads, rubbing their cheeks, licking themselves or vocalizing. About 1/3 of adult cats react more passively to nepetalactones, and may assume a sphinx-like posture, decrease vocalization or decrease movement. The effects of nepetalactones are similar in other Felidae.[2]
Mechanism of action
Felidae olfactory receptor exposure to nepetalactones or nepetalactols induces β-endorphin secretion to blood. This endorphin activates µ-opioid receptors as an agonist, thus working in a similar manner as morphine or other opioids.[5] Naloxone, a µ-opioid receptor antagonist, is known to block the effects of nepetalactones and nepetalactols in domestic cats, which supports this endorphin related mechanism of action.[2][5] Repeated exposure to nepetalactones or nepetalactols does not induce opioid withdrawals in Felidae, probably because endogenous β-endorphin secretion is controlled.[5] (cis,trans)- and (trans,cis)-nepetalactones have both been shown to function as cat attractants in domestic cats in studies of poor quality. Both isomers occur in catnip for example, but the (cis,trans)-isomer is the major one.[8]
Evolutionary reasons for the effects
Felidae react to plants that contain nepetalactones by licking them and rubbing them in their fur.[5] Nepetalactones and nepetalactols repel some disease causing insects. For example, nepetalactols are able to repel Aedes albopictus.[5][1] Felidae typically hunt other animals by stalking them. This requires being still or slow movements, which allow insects to bite Felidae more easily. This would make evolutionary pressure select for the behavior of rubbing of natural insect repellent. This was proposed to be the reason for this widely preserved behavioral trait in Felidae in a paper published in 2021.[5]
Sources
- 1 2 Lichman BR, et al. (2020). "The evolutionary origins of the cat attractant nepetalactone in catnip". Science Advances. 6 (20). doi:10.1126/sciadv.aba0721. PMC 7220310. PMID 32426505.
- 1 2 3 4 5 Espín-Iturbe LT, et al. (2017). "Active and passive responses to catnip (Nepeta cataria) are affected by age, sex and early gonadectomy in male and female cats". Behavioural Processes. 142: 110–115. doi:10.1016/j.beproc.2017.06.008. PMID 28698045.
- ↑ McElvain SM, Bright RD, Johnson PR (1941). "The constituents of the volatile oil of catnip. I. nepetalic acid, nepetalactone and related compounds". Journal of the American Chemical Society. 63 (6): 1558–1563. doi:10.1021/ja01851a019.
- ↑ Zimmermann N, et al. (2012). "Stereoselective synthesis of trans-fused iridoid lactones and their identification in the parasitoid wasp Alloxysta victrix, Part I: Dihydronepetalactones". Beilstein Journal of Organic Chemistry. 8 (1): 1246–1255. doi:10.3762/bjoc.8.140. PMC 3458745. PMID 23019455.
- 1 2 3 4 5 6 7 8 9 Uenoyama R, et al. (2021). "The characteristic response of domestic cats to plant iridoids allows them to gain chemical defense against mosquitoes". Science Advances. 7 (4). doi:10.1126/sciadv.abd9135. PMC 7817105.
- ↑ Haynes WM, et al. (2014). "3. Physical constants of organic compounds". CRC handbook of chemistry and physics (95th ed.). CRC Press. pp. 408–409. ISBN 9781482208689.
- ↑ Sherden NH, et al. (2018). "Identification of iridoid synthases from Nepeta species: Iridoid cyclization does not determine nepetalactone stereochemistry". Phytochemistry. 145: 48–56. doi:10.1016/j.phytochem.2017.10.004. PMC 5739345. PMID 29091815.
- 1 2 Bol S, et al. (2017). "Responsiveness of cats (Felidae) to silver vine (Actinidia polygama), Tatarian honeysuckle (Lonicera tatarica), valerian (Valeriana officinalis) and catnip (Nepeta cataria)". BMC Veterinary Research. 13. doi:10.1186/s12917-017-0987-6. PMC 5356310. PMID 28302120.
- ↑ Döring TF (2014). "How aphids find their host plants, and how they don't". Annals of Applied Biology. 165 (1): 3–26. doi:10.1111/aab.12142.
- 1 2 Hill JO, et al. (1976). "Species-characteristic responses to catnip by undomesticated felids". Journal of Chemical Ecology. 2 (2): 239–253. doi:10.1007/BF00987747.
- ↑ Resende LS, et al. (2011). "Influence of cinnamon and catnip on the stereotypical pacing of oncilla cats (Leopardus tigrinus) in captivity". Journal of applied animal welfare science. 14 (3): 247–254. doi:10.1080/10888705.2011.576981. PMID 22044295.