α-Aminoadipate pathway
The α-aminoadipate pathway is a biochemical pathway for the synthesis of the amino acid L-lysine. In the eukaryotes, this pathway is unique to the higher fungi (containing chitin in their cell walls) and the euglenids.[1] It has also been reported from bacteria of the genus Thermus.[2]
Pathway overview
Homocitrate is initially synthesised from acetyl-CoA and 2-oxoglutarate by homocitrate synthase. This is then converted to homoaconitate by homoaconitase and then to homoisocitrate by homoisocitrate dehydrogenase. A nitrogen atom is added from glutamate by aminoadipate aminotransferase to form the α-aminoadipate from which this pathway gets its name. This is then reduced by aminoadipate reductase via an acyl-enzyme intermediate to a semialdehyde. Reaction with glutamate by one class of saccharopine dehydrogenase yields saccharopine which is then cleaved by a second saccharopine dehydrogenase to yield lysine and oxoglutarate.[3]
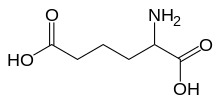
α-Aminoadipic acid
 | |
| Names | |
|---|---|
| IUPAC name
2-Aminohexanedioic acid | |
| Other names
2-Aminoadipic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| MeSH | 2-Aminoadipic+Acid |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C6H11NO4 | |
| Molar mass | 161.156 g/mol |
| Appearance | Crystalline |
| Density | 1.333 g/mL |
| Melting point | 196 °C (385 °F; 469 K) |
| Boiling point | 364 °C (687 °F; 637 K) |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
Irritant |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
α-Aminoadipic acid is the conjugate acid of α-aminoadipate, the latter of which is the prevalent form at physiological pH. A 2013 study identified α-Aminoadipic acid (2-aminoadipic acid) as a novel predictor of the development of diabetes and suggested that it is a potential modulator of glucose homeostasis in humans.[4]
See also
References
- Zabriskie TM, Jackson MD (2000). "Lysine biosynthesis and metabolism in fungi". Natural Product Reports. 17 (1): 85–97. doi:10.1039/a801345d. PMID 10714900.
- Kosuge T, Hoshino T (1999). "The α-aminoadipate pathway for lysine biosynthesis is widely distributed among Thermus strains". Journal of Bioscience and Bioengineering. 88 (6): 672–5. doi:10.1016/S1389-1723(00)87099-1. PMID 16232683.
- Xu H, Andi B, Qian J, West AH, Cook PF (2006). "The α-aminoadipate pathway for lysine biosynthesis in fungi". Cell Biochemistry and Biophysics. 46 (1): 43–64. doi:10.1385/CBB:46:1:43. PMID 16943623. S2CID 22370361.
- Wang TJ, Ngo D, Psychogios N, Dejam A, Larson MG, Vasan RS, Ghorbani A, O'Sullivan J, Cheng S, Rhee EP, Sinha S, McCabe E, Fox CS, O'Donnell CJ, Ho JE, Florez JC, Magnusson M, Pierce KA, Souza AL, Yu Y, Carter C, Light PE, Melander O, Clish CB, Gerszten RE (2013). "2-Aminoadipic acid is a biomarker for diabetes risk". Journal of Clinical Investigation. 123 (10): 4309–4317. doi:10.1172/JCI64801. PMC 3784523. PMID 24091325.