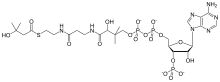
beta-Hydroxy beta-methylbutyryl-CoA
 | |
| Names | |
|---|---|
| IUPAC name
3′-O-Phosphonoadenosine 5′-[(3R)-3-hydroxy-4-{[3-({2-[(3-hydroxy-3-methylbutanoyl)sulfanyl]ethyl}amino)-3-oxopropyl]amino}-2,2-dimethyl-4-oxobutyl dihydrogen diphosphate] | |
| Systematic IUPAC name
O1-{[(2R,3S,4R,5R)-5-(6-Amino-9H-purin-9-yl)-4-hydroxy-3-(phosphonooxy)oxolan-2-yl]methyl} O3-[(3R)-3-hydroxy-4-{[3-({2-[(3-hydroxy-3-methylbutanoyl)sulfanyl]ethyl}amino)-3-oxopropyl]amino}-2,2-dimethyl-4-oxobutyl] dihydrogen diphosphate | |
| Other names
β-hydroxyisovaleryl-CoA 3-hydroxyisovaleryl-CoA 3-hydroxy-3-methylbutyryl-CoA | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID |
|
| |
| |
| Properties | |
| C26H44N7O18P3S | |
| Molar mass | 867.649946 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
β-Hydroxy β-methylbutyryl-coenzyme A (HMB-CoA), also known as 3-hydroxyisovaleryl-CoA, is a metabolite of L-leucine that is produced in the human body.[1][2] Its immediate precursors are β-hydroxy β-methylbutyric acid (HMB) and β-methylcrotonoyl-CoA (MC-CoA). It can be metabolized into HMB, MC-CoA, and HMG-CoA in humans.
Metabolic pathway
Notes
- This reaction is catalyzed by an unknown thioesterase enzyme.[3][4]
References
- Wilson JM, Fitschen PJ, Campbell B, Wilson GJ, Zanchi N, Taylor L, Wilborn C, Kalman DS, Stout JR, Hoffman JR, Ziegenfuss TN, Lopez HL, Kreider RB, Smith-Ryan AE, Antonio J (February 2013). "International Society of Sports Nutrition Position Stand: beta-hydroxy-beta-methylbutyrate (HMB)". Journal of the International Society of Sports Nutrition. 10 (1): 6. doi:10.1186/1550-2783-10-6. PMC 3568064. PMID 23374455.
- Kohlmeier M (May 2015). "Leucine". Nutrient Metabolism: Structures, Functions, and Genes (2nd ed.). Academic Press. pp. 385–388. ISBN 978-0-12-387784-0. Retrieved 6 June 2016.
Energy fuel: Eventually, most Leu is broken down, providing about 6.0kcal/g. About 60% of ingested Leu is oxidized within a few hours ... Ketogenesis: A significant proportion (40% of an ingested dose) is converted into acetyl-CoA and thereby contributes to the synthesis of ketones, steroids, fatty acids, and other compounds
Figure 8.57: Metabolism of L-leucine - "KEGG Reaction: R10759". Kyoto Encyclopedia of Genes and Genomes. Kanehisa Laboratories. Retrieved 24 June 2016.
- Mock DM, Stratton SL, Horvath TD, Bogusiewicz A, Matthews NI, Henrich CL, Dawson AM, Spencer HJ, Owen SN, Boysen G, Moran JH (November 2011). "Urinary excretion of 3-hydroxyisovaleric acid and 3-hydroxyisovaleryl carnitine increases in response to a leucine challenge in marginally biotin-deficient humans". primary source. The Journal of Nutrition. 141 (11): 1925–1930. doi:10.3945/jn.111.146126. PMC 3192457. PMID 21918059.
Metabolic impairment diverts methylcrotonyl CoA to 3-hydroxyisovaleryl CoA in a reaction catalyzed by enoyl-CoA hydratase (22, 23). 3-Hydroxyisovaleryl CoA accumulation can inhibit cellular respiration either directly or via effects on the ratios of acyl CoA:free CoA if further metabolism and detoxification of 3-hydroxyisovaleryl CoA does not occur (22). The transfer to carnitine by 4 carnitine acyl-CoA transferases distributed in subcellular compartments likely serves as an important reservoir for acyl moieties (39–41). 3-Hydroxyisovaleryl CoA is likely detoxified by carnitine acetyltransferase producing 3HIA-carnitine, which is transported across the inner mitochondrial membrane (and hence effectively out of the mitochondria) via carnitine-acylcarnitine translocase (39). 3HIA-carnitine is thought to be either directly deacylated by a hydrolase to 3HIA or to undergo a second CoA exchange to again form 3-hydroxyisovaleryl CoA followed by release of 3HIA and free CoA by a thioesterase.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.