(S)-mandelate dehydrogenase
In enzymology, (S)-mandelate dehydrogenase (EC 1.1.99.31) (MDH), is an enzyme that catalyzes the chemical reaction.
-Mandelate_Chemical_Structure.png.webp) + acceptor + acceptor |
= | 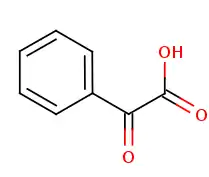 + reduced acceptor + reduced acceptor |
|---|---|---|
| (S)-Mandelate | 2-oxo-2-phenylacetate | |
| (S)-2-hydroxy-2-phenylacetate + acceptor ⇌ 2-oxo-2-phenylacetate + reduced acceptor | ||
| (S)-mandelate dehydrogenase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC no. | 1.1.99.31 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| |||||||||
Thus, the two substrates of this enzyme are (S)-2-hydroxy-2-phenylacetate and acceptor, whereas its two products are 2-oxo-2-phenylacetate and reduced acceptor.
This enzyme belongs to the family of oxidoreductases, specifically those acting on the CH-OH group of donor with other acceptors. The systematic name of this enzyme class is (S)-2-hydroxy-2-phenylacetate:acceptor 2-oxidoreductase.
This enzyme transfers the electron pair from FMNH2 to a component of the electron transport chain, most probably ubiquinone [1,2]. It is part of a metabolic pathway in Pseudomonads that allows these organisms to utilize mandelic acid, derivatized from the common soil metabolite amygdalin, as the sole source of carbon and energy. The enzyme has a large active-site pocket and preferentially binds substrates with longer sidechains, e.g. 2-hydroxyoctanoate rather than 2-hydroxybutyrate. It also prefers substrates that, like (S)-mandelate, have beta unsaturation, e.g. (indol-3-yl)glycolate compared with (indol-3-yl)lactate. Esters of mandelate, such as methyl (S)-mandelate, are also substrates.[1]
Synonyms
(S)-mandelate dehydrogenase is also knows as: L-mandelate dehydrogenase, L-MDH, MDH, SManDH, and SMDH.[1]
References
- "Information on EC 1.1.99.31 - (S)-mandelate dehydrogenase". Archived from the original on 2015-09-08.
- Lehoux IE, Mitra B (1999). "(S)-Mandelate dehydrogenase from Pseudomonas putida: mechanistic studies with alternate substrates and pH and kinetic isotope effects". Biochemistry. 38 (18): 5836–48. doi:10.1021/bi990024m. PMID 10231535.
- Dewanti AR, Xu Y, Mitra B (2004). "Role of glycine 81 in (S)-mandelate dehydrogenase from Pseudomonas putida in substrate specificity and oxidase activity". Biochemistry. 43 (33): 10692–700. doi:10.1021/bi049005p. PMID 15311930.
- Dewanti AR, Xu Y, Mitra B (2004). "Esters of mandelic acid as substrates for (S)-mandelate dehydrogenase from Pseudomonas putida: implications for the reaction mechanism". Biochemistry. 43 (7): 1883–90. doi:10.1021/bi036021y. PMID 14967029.