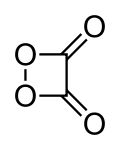
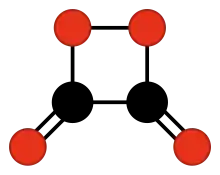
1,2-Dioxetanedione
The chemical compound 1,2-dioxetanedione, or 1,2-dioxacyclobutane-3,4-dione, often called peroxyacid ester, is an unstable oxide of carbon (an oxocarbon) with formula C2O4. It can be viewed as a double ketone of 1,2-dioxetane (1,2-dioxacyclobutane), or a cyclic dimer of carbon dioxide.[1]
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
1,2-Dioxetanedione | |
| Other names
Peroxyacid ester | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C2O4 | |
| Molar mass | 88.018 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
In ordinary conditions, it quickly decomposes to carbon dioxide (CO2) even at 180 K (−93.1 °C), but can be detected by mass spectrometry and other techniques.[2][3]
1,2-Dioxetanedione is an intermediate in the chemoluminescent reactions used in glowsticks.[4][5] The decomposition proceeds via a paramagnetic oxalate biradical intermediate.[6]
Recently it has been found that a high-energy intermediate in one of these reactions (between oxalyl chloride and hydrogen peroxide in ethyl acetate), which is presumed to be 1,2-dioxetanedione, can accumulate in solution at room temperature (up to a few micromoles at least), provided that the activating dye and all traces of metals and other reducing agents are removed from the system, and the reactions are carried out in an inert atmosphere.[7]
See also
References
- Alfred Hassner (1985): Chemistry of Heterocyclic Compounds: Small Ring Heterocycles, Part 3: Oxiranes, Arene Oxides, Oxaziridines, Dioxetanes, Thietanes, Thietes, Thiazetes, and Others, Volume 42. ISBN 978-0-471-05624-9 ISBN 978-0-470-18720-3 John Wiley & Sons.
- Herman F. Cordes; Herbert P. Richter; Carl A. Heller (1969). "Mass spectrometric evidence for the existence of 1,2-dioxetanedione (carbon dioxide dimer). Chemiluminescent intermediate". J. Am. Chem. Soc. 91 (25): 7209. doi:10.1021/ja01053a065.
- J. Stauff; W. Jaeschke; G. Schlögl (1972). "Chemilumineszenz des "Dioxetandions"" [Chemiluminescence of „Dioxetanedione“]. Z. Naturforsch. B 27 (11): 1434–1435. doi:10.1515/znb-1972-1140. S2CID 94708095.
- Vacher, Morgane; Fdez. Galván, Ignacio; Ding, Bo-Wen; Schramm, Stefan; Berraud-Pache, Romain; Naumov, Panče; Ferré, Nicolas; Liu, Ya-Jun; Navizet, Isabelle; Roca-Sanjuán, Daniel; Baader, Wilhelm J.; Lindh, Roland (March 2018). "Chemi- and Bioluminescence of Cyclic Peroxides". Chemical Reviews. 118 (15): 6927–6974. doi:10.1021/acs.chemrev.7b00649. PMID 29493234.
- Richard Bos; Neil W. Barnett; Gail A. Dyson; Kieran F. Lim; Richard A. Russell & Simon P. Watson (2003). "Studies on the mechanism of the peroxyoxalate chemiluminescence reaction: Part 1. Confirmation of 1,2-dioxetanedione as an intermediate using 13C nuclear magnetic resonance spectroscopy". Analytica Chimica Acta. 502 (2): 141–147. doi:10.1016/j.aca.2003.10.014.
- Richard Bos; Sarah A. Tonkin; Graeme R. Hanson; Christopher M. Hindson; Kieran F. Lim & Neil W. Barnett (2009). "In Search of a Chemiluminescence 1,4-Dioxy Biradical". J. Am. Chem. Soc. 131 (8): 2770–2771. doi:10.1021/ja808401p. PMID 19206238.
- Luiz F. M. L. Ciscato, Fernando H. Bartoloni, Erick L. Bastos, and Wilhelm J. Baader (2009), Direct Kinetic Observation of the Chemiexcitation Step in Peroxyoxalate Chemiluminescence. Journal of Organic Chemistry, volume 74, 8974–8979. doi:10.1021/jo901402k.
External links
 Media related to 1,2-Dioxetanedione at Wikimedia Commons
Media related to 1,2-Dioxetanedione at Wikimedia Commons