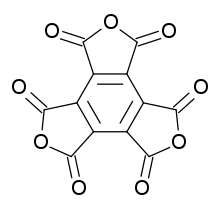
Mellitic anhydride
Mellitic anhydride, the anhydride of mellitic acid, is an organic compound with the formula C12O9.
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Benzo[1,2-c:3,4-c′:5,6-c′′]trifuran-1,3,4,6,7,9-hexone | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C12O9 | |
| Molar mass | 288.123 g·mol−1 |
| Appearance | colorless solid[1] |
| Melting point | 161 °C; 322 °F; 434 K[1] |
| Vapor pressure | 0.000004 mmHg (20°C)[1] |
| Hazards | |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
none[1] |
REL (Recommended) |
TWA 0.005 ppm (0.04 mg/m3) Should be handled in the workplace as an extremely toxic substance.[1] |
IDLH (Immediate danger) |
N.D.[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Containing no other elements (e.g., hydrogen) besides carbon and oxygen, mellitic anhydride is an oxide of carbon (oxocarbon), and, along with CO2, CO, and C3O2, is one of the only four that are reasonably stable under standard conditions. It is a white sublimable solid, apparently obtained by Justus Liebig and Friedrich Wöhler in 1830 in their study of mellite ("honey stone") and has the empirical formula C4O3.[2][3][4] The substance was properly characterized in 1913 by H. Meyer and K. Steiner.[5][6] It retains the aromatic character of the benzene ring.[7][8]
References
- NIOSH Pocket Guide to Chemical Hazards. "#0635". National Institute for Occupational Safety and Health (NIOSH).
- Wöhler, F. (1826). "Ueber die Honigsteinsäure". Annalen der Physik und Chemie. 83 (7): 325–334. Bibcode:1826AnP....83..325W. doi:10.1002/andp.18260830706.
- Liebig, J.; Wöhler, F. (1830). "Ueber die Zusammensetzung der Honigsteinsäure". Annalen der Physik und Chemie. 94 (2): 161–164. Bibcode:1830AnP....94..161L. doi:10.1002/andp.18300940202.
- Erdmann, O. L.; Marchand, R. F. (1848). "Ueber die Mellithsäure". Journal für Praktische Chemie. 43 (2/3): 129–144. doi:10.1002/prac.18480430113.
- Meyer, H.; Steiner, K. (1913). "Über ein neues Kohlenoxyd C12O9" [A new carbon oxide C12O9]. Berichte der Deutschen Chemischen Gesellschaft. 46 (1): 813–815. doi:10.1002/cber.191304601105.
- Bugge, G. (1914). "Chemie: Ein neues Kohlenoxyd". Naturwissenschaftliche Wochenschrift. 13/29 (12): 188.
- Fowler, P. W.; Lillington, M. (2007). "Mellitic Trianhydride, C12O9: The Aromatic Oxide of Carbon". Journal of Chemical Information and Modeling. 47 (3): 905–908. doi:10.1021/ci600547n. PMID 17315989.
- Ermer, O.; Neudörfl, J. (2000). "Structure of Mellitic Trianhydride". Helvetica Chimica Acta. 83 (1): 300–309. doi:10.1002/(SICI)1522-2675(20000119)83:1<300::AID-HLCA300>3.0.CO;2-L.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.