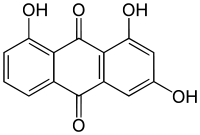
1,3,8-Trihydroxyanthraquinone
1,3,8-Trihydroxyanthraquinone is an organic compound. It is one of many trihydroxyanthraquinone isomers, formally derived from anthraquinone by replacement of three hydrogen atoms by hydroxyl (OH) groups.
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
1,3,8-Trihydroxyanthracene-9,10-dione | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C14H8O5 | |
| Molar mass | 256.210 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
The compound occurs in some microorganisms[1] and in alcoholic extracts of the wood of the South American plant Senna reticulata (mangerioba grande or maria mole in Portuguese), used in the local folk medicine for liver problems and rheumatism. The extract also contained, among other products chrysophanol (1,8-dihydroxy-3-methylanthraquinone), physcion (1,8-dihydroxy-3-methyl-6-methoxyanthraquinone), aloe-emodin (3-carbinol-1,8-dihydroxyanthraquinone), lunatin (3-methoxy-1,6,8-trihydroxyanthraquinone), emodin (6-methyl-1,3,8-trihydroxyanthraquinone), and chrysophanol-10,10'-bianthrone.[1]
The substance is soluble in ethanol and chloroform but not in n-hexane, and melts at 283 °C.[1]
References
- SANTOS, Rogério Nunes dos; SILVA, Maria Goretti de Vasconcelos, and BRAZ FILHO, Raimundo (2008). Constituintes químicos do caule de Senna reticulata Willd. (Leguminoseae) ("Chemical constituents isolated from the wood of Senna reticulata Willd") Química Nova [online], volume 31 issue 8, pages 1979--1981 (in Portuguese). doi:10.1590/S0100-40422008000800011 "It is the first report of 1,3,8-trihydroxyanthraquinone and 3-methoxy-1,6,8-trihydroxyanthraquinone in higher plants."