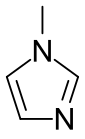
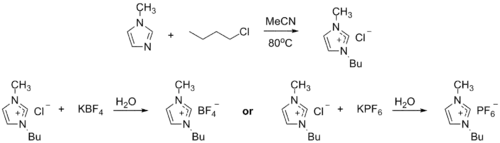1-Methylimidazole
1-Methylimidazole or N-methylimidazole is an aromatic heterocyclic organic compound with the formula CH3C3H3N2. It is a colourless liquid that is used as a specialty solvent, a base, and as a precursor to some ionic liquids. It is a fundamental nitrogen heterocycle and as such mimics for various nucleoside bases as well as histidine and histamine.
 | |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1-Methyl-1H-imidazole | |||
| Other names
1-Methylimidazole N-Methylimidazole NMI | |||
| Identifiers | |||
3D model (JSmol) |
|||
| 105197 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.009.532 | ||
| EC Number |
| ||
| 2403 | |||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C4H6N2 | |||
| Molar mass | 82.10 g/mol | ||
| Density | 1.03 g/cm3 | ||
| Melting point | −6 °C (21 °F; 267 K) | ||
| Boiling point | 198 °C (388 °F; 471 K) | ||
| Hazards | |||
| GHS labelling: | |||
  | |||
| Danger | |||
| H302, H312, H314 | |||
| P260, P264, P270, P280, P301+P312, P301+P330+P331, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P310, P312, P321, P322, P330, P363, P405, P501 | |||
| Safety data sheet (SDS) | Oxford MSDS | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
Basicity
With the N-methyl group, this particular derivative of imidazole cannot tautomerize. It is slightly more basic than imidazole, as indicated by the pKa's of the conjugate acids of 7.0 and 7.4.[1] Methylation also provides a significantly lower melting point, which makes 1-methylimidazole a useful solvent.
Synthesis
1-Methylimidazole is prepared mainly by two routes industrially. The main one is acid-catalysed methylation of imidazole by methanol. The second method involves the Radziszewski reaction from glyoxal, formaldehyde, and a mixture of ammonia and methylamine.[2][3]
- (CHO)2 + CH2O + CH3NH2 + NH3 → H2C2N(NCH3)CH + 3 H2O
The compound can be synthesized on a laboratory scale by methylation of imidazole at the pyridine-like nitrogen and subsequent deprotonation.[4] Similarly, 1-methylimidazole may be synthesized by first deprotonating imidazole to form a sodium salt followed by methylation.[5][6]
- H2C2N(NH)CH + CH3I → [H2C2(NH)(NCH3)CH]I
- [H2C2(NH)(NCH3)CH]I + NaOH → H2C2N(NCH3)CH + H2O + NaI
Applications
In the research laboratory, 1-methylimidazole and related derivatives have been used as mimic aspects of diverse imidazole-based biomolecules.
1-Methylimidazole is also the precursor for the synthesis of the methylimidazole monomer of pyrrole-imidazole polyamides. These polymers can selectively bind specific sequences of double-stranded DNA by intercalating in a sequence dependent manner.[7]
Ionic liquid precursor
1-Methylimidazole alkylates to form dialkyl imidazolium salts. Depending on the alkylating agent and the counteranion, various ionic liquids result, e.g. 1-butyl-3-methylimidazolium hexafluorophosphate ("BMIMPF6"):[8][9]
BASF has used 1-methylimidazole as a means to remove acid during their industrial-scale production of diethoxyphenylphosphine. In this biphasic acid scavenging using ionic liquids (BASIL) process, 1-methylimidazole reacts with HCl to produce 1-methylimidazolium hydrochloride, which spontaneously separates as a separate liquid phase under the reaction conditions.[8][10]
- 2 MeC3N2H3 + C6H5PCl2 + 2 C2H5OH → 2 [MeC3N2H4]Cl + C6H5P(OC2H5)2
Donor properties
1-methylimidazole (NMIz) as a ligand forms octahedral ions M(NMIz)62+with M = Fe, Co, Ni, and a square-planar ion Cu(NMIz)42+. [11] 1-methylimidazole forms adducts with Lewis acids such as molybdenum perfluorobutyrate and [Rh(CO)2Cl]2. The donor properties of 1-methylimidazole have been analyzed by the ECW model yielding EB= 1.16 and CB= 4.92.
See also
References
- Albert, A., Heterocyclic Chemistry, 2nd ed.; 1968 Athlone Press, ISBN 0-485-11092-X
- Ebel, K.; Koehler, H.; Gamer, A. O. & Jäckh, R. (2002). "Imidazole and Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a13_661.
- Bronislaw Radziszewski (1882). "Ueber die Constitution des Lophins und verwandter Verbindungen" [By the Constitution of the Lophins and related compounds]. Berichte der deutschen chemischen Gesellschaft (in German). 15 (2): 1493–1496. doi:10.1002/cber.18820150207.
- Gilchrist, T. L., Heterocyclic Chemistry, 2nd ed.; 1992 Longman Scientific & Technical, ISBN 0-582-06420-1
- Grimmett, M. R., Imidazole and Benzimidazole Synthesis; 1997 Academic Press, ISBN 0-12-303190-7
- Gupta, R. R., Kumar, M., Gupta, V., Heterocyclic Chemistry II: Five Membered Heterocycles; 1999 Springer, ISBN 3-540-65252-3
- Baird, Eldon E.; Dervan, Peter B. (1996). "Solid Phase Synthesis of Polyamides Containing Imidazole and Pyrrole Amino Acids" (PDF). Journal of the American Chemical Society. 118 (26): 6141–6. doi:10.1021/ja960720z.
- Meindersma, G. Wytze; Maase, Matthias; De Haan, André B. (2007). "Ionic Liquids". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.l14_l01. ISBN 978-3-527-30673-2.
- Dupont, J.; Consorti, C.; Suarez, P.; de Souza, R. (2002). "Preparation of 1-Butyl-3-methyl imidazolium-based Room Temperature Ionic Liquids". Organic Syntheses. 79: 236. doi:10.15227/orgsyn.079.023.
- Welton, Tom (11 November 2015). "Solvents and sustainable chemistry". Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences. 471 (2183): 20150502. doi:10.1098/rspa.2015.0502. PMC 4685879. PMID 26730217.

- Reedijk,R. (1969). "Pyrazoles and imidazoles as ligands. II. Coordination compounds of N-methyl imidazole with metal perchlorates and tetrafluoroborates". Inorganica Chimica Acta. 3: 517–522. doi:10.1016/S0020-1693(00)92544-1.