Acetylacetone
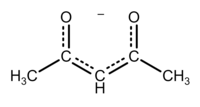
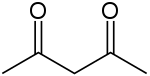
Acetylacetone is an organic compound with the chemical formula CH3COCH2COCH3. It is a colorless liquid, classified as a 1,3-diketone. It exists in equilibrium with a tautomer CH3C(O)CH=C(OH)CH3. These tautomers interconvert so rapidly under most conditions that they are treated as a single compound in most applications.[2] It is a colorless liquid that is a precursor to acetylacetonate anion (commonly abbreviated acac−), a bidentate ligand. It is also a building block for the synthesis of heterocyclic compounds.
 | |||
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC names
(3Z)-4-Hydroxy-3-penten-2-one (enol form) Pentane-2,4-dione (keto form) | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol) |
|||
| 741937 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.004.214 | ||
| EC Number |
| ||
| 2537 | |||
| KEGG | |||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 2310 | ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C5H8O2 | |||
| Molar mass | 100.117 g·mol−1 | ||
| Density | 0.975 g/mL[1] | ||
| Melting point | −23 °C (−9 °F; 250 K) | ||
| Boiling point | 140 °C (284 °F; 413 K) | ||
| 16 g/100 mL | |||
| -54.88·10−6 cm3/mol | |||
| Hazards | |||
| GHS labelling: | |||
    | |||
| Danger | |||
| H226, H302, H311, H320, H331, H335, H341, H370, H412 | |||
| P201, P202, P210, P233, P240, P241, P242, P243, P260, P261, P264, P270, P271, P273, P280, P281, P301+P312, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P307+P311, P308+P313, P311, P312, P321, P322, P330, P337+P313, P361, P363, P370+P378, P403+P233, P403+P235, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 34 °C (93 °F; 307 K) | ||
| 340 °C (644 °F; 613 K) | |||
| Explosive limits | 2.4–11.6% | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
Properties
Tautomerism
| Solvent | Kketo→enol |
|---|---|
| Gas phase | 11.7 |
| Cyclohexane | 42 |
| Toluene | 10 |
| THF | 7.2 |
| CDCl3[3] | 5.7 |
| DMSO | 2 |
| Water | 0.23 |
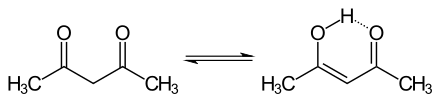
The keto and enol tautomers of acetylacetone coexist in solution. The enol form has C2v symmetry, meaning the hydrogen atom is shared equally between the two oxygen atoms.[4] In the gas phase, the equilibrium constant, Kketo→enol, is 11.7, favoring the enol form. The two tautomeric forms can be distinguished by NMR spectroscopy, IR spectroscopy and other methods.[5][6]
The equilibrium constant tends to be high in nonpolar solvents; when k = >1, the enol form is favoured. The keto form becomes more favourable in polar, hydrogen-bonding solvents, such as water.[7] The enol form is a vinylogous analogue of a carboxylic acid.
Acid–base properties
| solvent | T/°C | pKa[8] |
|---|---|---|
| 40% ethanol/water | 30 | 9.8 |
| 70% dioxane/water | 28 | 12.5 |
| 80% DMSO/water | 25 | 10.16 |
| DMSO | 25 | 13.41 |
Acetylacetone is a weak acid:
- C5H8O2 ⇌ C5H7O−2 + H+
IUPAC recommended pKa values for this equilibrium in aqueous solution at 25 °C are 8.99 ± 0.04 (I = 0), 8.83 ± 0.02 (I = 0.1 M NaClO4) and 9.00 ± 0.03 (I = 1.0 M NaClO4; I = Ionic strength).[9] Values for mixed solvents are available. Very strong bases, such as organolithium compounds, will deprotonate acetylacetone twice. The resulting dilithium species can then be alkylated at C-1.
Preparation
Acetylacetone is prepared industrially by the thermal rearrangement of isopropenyl acetate.[10]
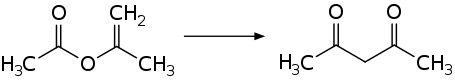
Laboratory routes to acetylacetone also begin with acetone. Acetone and acetic anhydride upon the addition of boron trifluoride (BF3) catalyst:[11]
- (CH3CO)2O + CH3C(O)CH3 → CH3C(O)CH2C(O)CH3
A second synthesis involves the base-catalyzed condensation of acetone and ethyl acetate, followed by acidification:[11]
- NaOEt + EtO2CCH3 + CH3C(O)CH3 → NaCH3C(O)CHC(O)CH3 + 2 EtOH
- NaCH3C(O)CHC(O)CH3 + HCl → CH3C(O)CH2C(O)CH3 + NaCl
Because of the ease of these syntheses, many analogues of acetylacetonates are known. Some examples include benzoylacetone, dibenzoylmethane (dbaH) and tert-butyl analogue tetramethyl-3,5-heptanedione. Trifluoroacetylacetone and hexafluoroacetylacetonate are also used to generate volatile metal complexes.
Reactions
Condensations
Acetylacetone is a versatile bifunctional precursor to heterocycles because both keto groups undergo condensation. Hydrazine reacts to produce pyrazoles. Urea gives pyrimidines. Condensation with two aryl- and alkylamines to gives NacNacs, wherein the oxygen atoms in acetylacetone are replaced by NR (R = aryl, alkyl).
Coordination chemistry

Sodium acetylacetonate, Na(acac), is the precursor to many acetylacetonate complexes. A general method of synthesis is to treat a metal salt with acetylacetone in the presence of a base:[12]
- MBz + z Hacac ⇌ M(acac)z + z BH
Both oxygen atoms bind to the metal to form a six-membered chelate ring. In some cases the chelate effect is so strong that no added base is needed to form the complex.
Biodegradation
The enzyme acetylacetone dioxygenase cleaves the carbon-carbon bond of acetylacetone, producing acetate and 2-oxopropanal. The enzyme is iron(II)-dependent, but it has been proven to bind to zinc as well. Acetylacetone degradation has been characterized in the bacterium Acinetobacter johnsonii.[13]
- C5H8O2 + O2 → C2H4O2 + C3H4O2
References
- "05581: Acetylacetone". Sigma-Aldrich.
- Thomas M. Harris (2001). "2,4-Pentanedione". 2,4‐Pentanedione. e-EROS Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rp030. ISBN 0471936235.
- Smith, Kyle T.; Young, Sherri C.; DeBlasio, James W.; Hamann, Christian S. (12 April 2016). "Measuring Structural and Electronic Effects on Keto–Enol Equilibrium in 1,3-Dicarbonyl Compounds". Journal of Chemical Education. 93 (4): 790–794. doi:10.1021/acs.jchemed.5b00170.
- Caminati, W.; Grabow, J.-U. (2006). "The C2v Structure of Enolic Acetylacetone". Journal of the American Chemical Society. 128 (3): 854–857. doi:10.1021/ja055333g. PMID 16417375.
- Manbeck, Kimberly A.; Boaz, Nicholas C.; Bair, Nathaniel C.; Sanders, Allix M. S.; Marsh, Anderson L. (2011). "Substituent Effects on Keto–Enol Equilibria Using NMR Spectroscopy". Journal of Chemical Education. 88 (10): 1444–1445. Bibcode:2011JChEd..88.1444M. doi:10.1021/ed1010932.
- Yoshida, Z.; Ogoshi, H.; Tokumitsu, T. (1970). "Intramolecular hydrogen bond in enol form of 3-substituted-2,4-pentanedione". Tetrahedron. 26 (24): 5691–5697. doi:10.1016/0040-4020(70)80005-9.
- Reichardt, Christian (2003). Solvents and Solvent Effects in Organic Chemistry (3rd ed.). Wiley-VCH. ISBN 3-527-30618-8.
- IUPAC SC-Database Archived 2017-06-19 at the Wayback Machine A comprehensive database of published data on equilibrium constants of metal complexes and ligands
- Stary, J.; Liljenzin, J. O. (1982). "Critical evaluation of equilibrium constants involving acetylacetone and its metal chelates" (PDF). Pure and Applied Chemistry. 54 (12): 2557–2592. doi:10.1351/pac198254122557. S2CID 96848983.
- Siegel, Hardo; Eggersdorfer, Manfred (2002). "Ketones". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a15_077. ISBN 9783527306732.
- Denoon, C. E., Jr.; Adkins, Homer; Rainey, James L. (1940). "Acetylacetone". Organic Syntheses. 20: 6. doi:10.15227/orgsyn.020.0006.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - O'Brien, Brian. "Co(tfa)3 & Co(acac)3 handout" (PDF). Gustavus Adolphus College.
- Straganz, G.D.; Glieder, A.; Brecker, L.; Ribbons, D.W.; Steiner, W. (2003). "Acetylacetone-cleaving enzyme Dke1: a novel C–C-bond-cleaving enzyme from Acinetobacter johnsonii". Biochemical Journal. 369 (3): 573–581. doi:10.1042/BJ20021047. PMC 1223103. PMID 12379146.