Barium acetylacetonate
Barium acetylacetonate is a compound with formula Ba(C5H7O2)2. It is the Ba2+ complex of the anion acetylacetonate. The compound is typically encountered as an ill-defined hydrate, which would accord with the high coordination number characteristic of barium.[1][2]
| Names | |
|---|---|
| IUPAC name
Barium(2+); (Z)-4-oxopent-2-en-2-olate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.031.944 |
| EC Number |
|
PubChem CID |
|
| |
| |
| Properties | |
| C10H14BaO4 | |
| Molar mass | 335.545 g·mol−1 |
| Appearance | White solid |
| Hazards | |
| GHS labelling: | |
 | |
| Warning | |
| H302, H332 | |
| P261, P264, P270, P271, P301+P312, P304+P312, P304+P340, P312, P330, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Uses
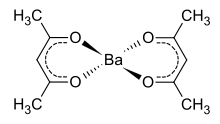 Structure of gaseous barium acetylacetonate.
Structure of gaseous barium acetylacetonate.
Barium acetylacetonate has been examined in metal organic chemical vapour deposition of BaTiO3 thin films.[3] The related complex with hexafluoroacetylacetonate Ba(hfa)2(tetraglyme) has also been investigated. Its formation of a sublimable adduct containing a polyether illustrates the high coordination numbers typical of barium.[4]
References
- Paw, Witold; Baum, Thomas H.; Lam, Kin-Chung; Rheingold, Arnold L. (2000). "Low-Melting, Mononuclear Tetrahydrofuran Complexes of M(2,2,6,6-tetramethylheptane-3,5-dionate)2 (M = Ba, Sr) and Related Analogues". Inorganic Chemistry. 39 (9): 2011–2014. doi:10.1021/ic990641z. PMID 11428125.
- Rossetto, G.; Polo, A.; Benetollo, F.; Porchia, M.; Zanella, P. (1992). "Studies on molecular barium precursors for MOCVD: Synthesis and characterization of barium 2,2,6,6-tetramethyl-3,5-heptanedionate. X-ray crystal structure of [Ba(THD)2·Et2O]2". Polyhedron. 11 (8): 979–985. doi:10.1016/S0277-5387(00)83351-3.
- Lee, C. H.; Park, S. J. (1990-12-01). "Preparation of ferroelectric BaTiO3 thin films by metal organic chemical vapour deposition". Journal of Materials Science: Materials in Electronics. 1 (4): 219–224. doi:10.1007/BF00696081. S2CID 101586516.
- Schulz, Douglas L.; Neumayer, Deborah A.; Marks, Tobin J. (1997). "Volatile β-Diketonate Complexes of Calcium(II), Strontium(II), and Barium(II)". Inorganic Syntheses. 31: 1–7. doi:10.1002/9780470132623.ch1. ISBN 9780470132623.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.