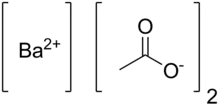
Barium acetate
Barium acetate (Ba(C2H3O2)2) is the salt of barium(II) and acetic acid. Barium acetate is toxic to humans, but has use in chemistry and manufacturing.
 | |
| Names | |
|---|---|
| IUPAC name
Barium acetate | |
| Other names
Barium diacetate | |
| Identifiers | |
3D model (JSmol) |
|
| Abbreviations | Ba(OAc)2 |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.008.045 |
| EC Number |
|
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4H6BaO4 | |
| Molar mass | 255.415 g·mol−1 |
| Appearance | White solid |
| Odor | odorless |
| Density | 2.468 g/cm3 (anhydrous) 2.19 g/cm3 (monohydrate) |
| Melting point | 450 °C (842 °F; 723 K) decomposes |
| 55.8 g/100 mL (0 °C) 72 g/100mL (20 °C) | |
| Solubility | slightly soluble in ethanol, methanol |
| -100.1·10−6 cm3/mol (2H2O) | |
| Structure | |
| tetragonal | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
Toxic, hazardous on ingestion |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
108 mg/kg (oral, rat) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Preparation
Barium acetate is generally produced by the reaction of acetic acid with barium carbonate:[2]
The reaction is performed in solution and the barium acetate crystalizes out at temperatures above 41 °C. Between 25 and 40 °C, the monohydrate version crystalizes. Alternatively, barium sulfide can be used:[2]
Again, the solvent is evaporated off and the barium acetate crystallized.
Properties
Barium acetate is a white powder, which is highly soluble: at 0 °C, 55.8 g of barium acetate can be dissolved in 100 g of water. It decomposes upon heating into barium carbonate.
Reactions
When heated in air, barium acetate decomposes to the carbonate. It reacts with acids: reaction with sulfuric acid, hydrochloric acid and nitric acid give the sulfate, chloride and nitrate respectively.
Uses
Barium acetate is used as a mordant for printing textile fabrics, for drying paints and varnishes and in lubricating oil. In chemistry, it is used in the preparation of other acetates; and as a catalyst in organic synthesis.
In pop culture
Barium Acetate was featured in a 2001 episode of the television series Forensic Files, recounting the 1993 murder of a man by his teenage daughter (Marie Robards), though the episode and other crime documentary shows examining the Robards case excluded the mention of barium acetate.
Barium Acetate was featured in a 2014 episode of the crime documentary series Redrum.
Barium acetate was named as the choice poison of a teen's murder of her father in Deadly Women "Parents Peril", S6 E2.
References
- , JT Baker
- Barium acetate Archived June 28, 2009, at the Wayback Machine, hillakomem.com, retrieved 30 June 2009
Further reading
- I. Gautier-Luneau; A. Mosset (1988). "Crystal structure of anhydrous barium acetate". Journal of Solid State Chemistry. 73 (2): 473–479. Bibcode:1988JSSCh..73..473G. doi:10.1016/0022-4596(88)90133-8.
- After husband's body was found burned, woman is suspected of poisoning another man
