Barium thiocyanate
Barium thiocyanate is a colorless water-soluble salt that is very hygroscopic. It is highly toxic to ingestion and irritates the skin. It is also soluble in most alcohols and insoluble in simple alkanes.
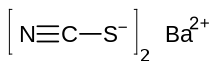 | |
| Names | |
|---|---|
| IUPAC name
Barium thiocyanate | |
Other names
| |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.016.587 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| Ba(SCN)2 | |
| Molar mass | 253.49 g/mol |
| Appearance | White crystals |
| 62.63 g/100 ml (25°C) | |
| Solubility | Soluble in acetone, methanol, and ethanol |
| Hazards | |
| GHS labelling: | |
 | |
| H301, H312, H315, H319, H332, H335 | |
| P261, P280, P302+P352, P304+P340 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Uses
Barium thiocyanate is used in dyeing textiles and is an ingredient in some photographic solutions. But because of its toxicity, it has limited uses.[3]
Preparation
Barium thiocyanate is prepared by dissolving barium metal or barium nitrate in a solution of thiocyanic acid.
References
- "Barium thiocyanate | 336879-43-7". Sigma-Aldrich. 2012-09-14. Retrieved 2021-01-20.
- "BARIUM THIOCYANATE | 2092-17-3". Chemicalbook.com. 2020-07-03. Retrieved 2021-01-20.
- "Barium thiocyanate - CAMEO". Cameo.mfa.org. 2016-04-29. Retrieved 2021-01-20.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.