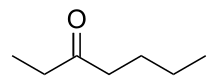
3-Heptanone
3-Heptanone (butyl ethyl ketone), is a seven carbon ketone. It is a colorless liquid with a "green odor," also described to have a fruity scent. It is often used as a perfume/fragrance, as a solvent for cellulose, nitrocellulose, or vinyl resins, and as a synthetic building block in the preparation of other organic molecules.[4]
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Heptan-3-one | |
| Other names
Ethyl butyl ketone 3-Oxoheptane Butyl ethyl ketone | |
| Identifiers | |
3D model (JSmol) |
|
| 506161 | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.003.081 |
| EC Number |
|
| MeSH | 3-Heptanone |
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 1224 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C7H14O | |
| Molar mass | 114.188 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | powerful, fruity[1] |
| Density | 0.812 g cm−3 |
| Melting point | −39 °C (−38 °F; 234 K) |
| Boiling point | 146 to 149 °C (295 to 300 °F; 419 to 422 K) |
| 1% (20 °C)[1] | |
| Vapor pressure | 4 mmHg (20 °C)[1] |
| Hazards | |
| GHS labelling: | |
  | |
| Warning | |
| H226, H319, H332 | |
| P210, P233, P240, P241, P242, P243, P261, P264, P271, P280, P303+P361+P353, P304+P312, P304+P340, P305+P351+P338, P312, P337+P313, P370+P378, P403+P235, P501 | |
| Flash point | 41 °C (106 °F; 314 K) |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
2760 mg/kg (rat, oral)[2] |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
TWA 50 ppm (230 mg/m3)[1] |
REL (Recommended) |
TWA 50 ppm (230 mg/m3)[1] |
IDLH (Immediate danger) |
1000 ppm[1] |
| Safety data sheet (SDS) | [3] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Preparation
3-Heptanone is produced industrially through reductive condensation of propionaldehyde (propanal) with butanone (methyl ethyl ketone). This reaction yields hept-4-en-3-one, which is subsequently hydrogenated to 3-heptanone.
- CH
3CH
2CHO + CH
3C(O)CH
2CH
3 → CH
3CH
2C(O)CHCHCH
2CH
3 + H
2O
- CH
3CH
2C(O)CHCHCH
2CH
3 + H
2 → CH
3CH
2C(O)CH
2CH
2CH
2CH
3
References
- NIOSH Pocket Guide to Chemical Hazards. "#0266". National Institute for Occupational Safety and Health (NIOSH).
- "Ethyl butyl ketone". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- http://www.chemicalbook.com/ProductMSDSDetailCB0852672_EN.htm External MSDS
- Siegel, Hardo; Eggersdorfer, Manfred (2012). "Ketones". Ullmann's Encyclopedia of Industrial Chemistry. Vol. 20. p. 195. doi:10.1002/14356007.a15_077. ISBN 9783527306732.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.