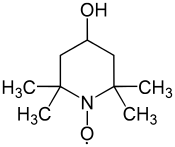
4-Hydroxy-TEMPO
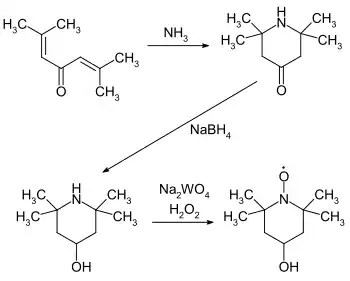
4-Hydroxy-TEMPO or TEMPOL, formally 4-hydroxy-2,2,6,6-tetramethylpiperidin-1-oxyl, is a heterocyclic compound. Like the related TEMPO, it is used as a catalyst and chemical oxidant by virtue of being a stable aminoxyl radical. Its major appeal over TEMPO is that it is less expensive, being produced from triacetone amine, which is itself made via the condensation of acetone and ammonia. This makes it economically viable on an industrial scale.[3]
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
(4-Hydroxy-2,2,6,6-tetramethylpiperidin-1-yl)oxyl | |
| Other names
tempol; tanol; TMPN; 4-Oxypiperidol; nitroxyl 2; HyTEMPO | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.017.056 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C9H18NO2 | |
| Molar mass | 172.248 g·mol−1 |
| Appearance | Orange crystals |
| Melting point | 71–73 °C (160–163 °F; 344–346 K)[1] |
| 629.3 g/L (20 °C) | |
| Hazards | |
| GHS labelling: | |
 [2] [2] | |
| Warning[2] | |
| H302, H315, H319, H335[2] | |
| P261, P305+P351+P338[2] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
In biochemical research, 4-hydroxy-TEMPO has been investigated as an agent for limiting reactive oxygen species. It catalyzes the disproportionation of superoxide, facilitates hydrogen peroxide metabolism, and inhibits Fenton chemistry.[4] 4-Hydroxy-TEMPO, along with related nitroxides, are being studied for their potential antioxidant properties.[5]
On an industrial-scale 4-hydroxy-TEMPO is often present as a structural element in hindered amine light stabilizers, which are commonly used stabilizers in plastics, it is also used as a polymerisation inhibitor, particularly during the purification of styrene.
It is a promising model substance to inhibit SARS-CoV-2 RNA-dependent RNA polymerase.[6]
References
- Zakrzewski, Jerzy; Krawczyk, Maria (1 January 2011). "Reactions of Nitroxides. Part XII [1]. – 2,2,6,6-Tetramethyl-1-oxyl- 4-piperidyl Chloroformate – A New Reactive Nitroxyl Radical. A One-pot Synthesis of 2,2,6,6-Tetramethyl-1-oxyl-4-piperidyl N,N-Dialkyl-carbamates". Zeitschrift für Naturforschung B. 66 (5): 493–498. doi:10.1515/znb-2011-0509. S2CID 51802316.
- Sigma-Aldrich Co., 4-Hydroxy-TEMPO. Retrieved on 2015-08-24.
- Ciriminna, Rosaria; Pagliaro, Mario (15 January 2010). "Industrial Oxidations with Organocatalyst TEMPO and Its Derivatives". Organic Process Research & Development. 14 (1): 245–251. doi:10.1021/op900059x.
- Wilcox, C. S.; Pearlman, A. (2008). "Chemistry and Antihypertensive Effects of Tempol and Other Nitroxides". Pharmacological Reviews. 60 (4): 418–69. doi:10.1124/pr.108.000240. PMC 2739999. PMID 19112152.
- Lewandowski, M; Gwozdzinski, K. (2017). "Nitroxides as Antioxidants and Anticancer Drugs". International Journal of Molecular Sciences. 18 (11): 2490. doi:10.3390/ijms18112490. PMC 5713456. PMID 29165366.
- Maio, N.; Lafont, B.A.P.; Sil, D.; Li, Y.; Bollinger, M.; Krebs, C. (2021). "Fe-S cofactors in the SARS-CoV-2 RNA-dependent RNA polymerase are potential antiviral targets". Science. 373 (6551): 236–241. doi:10.1126/science.abi5224. PMC 8892629. PMID 34083449.