Triacetonamine
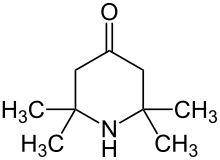
Triacetonamine is an organic compound with the formula OC(CH2CMe2)2NH (where Me = CH3). It is a colorless or white solid that melts near room temperature. The compound is an intermediate in the preparation of 2,2,6,6-tetramethylpiperidine, a sterically hindered base and precursor to the reagent called TEMPO. Triacetonamine is formed by the poly-aldol condensation of acetone in the presence of ammonia and calcium chloride:[1]
- 3 (CH3)2CO + NH3 → OC(CH2CMe2)2NH + 2 H2O
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2,2,6,6-Tetramethylpiperidin-4-one | |
| Other names
Triacetone amine | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.011.413 |
| EC Number |
|
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C9H17NO | |
| Molar mass | 155.241 g·mol−1 |
| Appearance | Colorless low-melting solid |
| Density | 0.882 g/cm3 |
| Melting point | 43 °C (109 °F; 316 K) |
| Boiling point | 205 °C (401 °F; 478 K) |
| Moderate | |
| Solubility in other solvents | Most organic solvents |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
flammable |
| GHS labelling: | |
  | |
| Danger | |
| H290, H302, H314, H315, H317, H319, H335, H412 | |
| P234, P260, P261, P264, P270, P271, P272, P273, P280, P301+P312, P301+P330+P331, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P310, P312, P321, P330, P332+P313, P333+P313, P337+P313, P362, P363, P390, P403+P233, P404, P405, P501 | |
| Flash point | 73 °C; 164 °F; 346 K |
| Related compounds | |
Related compounds |
Piperidine |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Reductive amination of triacetonamine gives 4-amino-2,2,6,6-tetramethylpiperidine.
It is primarily used as a stabilizer for plastics, typically via its conversion to number of hindered amine light stabilizers, but also finds use as a chemical feedstock. It is used to prepare the hindered amine 2,2,6,6-tetramethylpiperidine, CH2[CH2C(CH3)2]2NH,[2] as well as the radical oxidizer 4-Hydroxy-TEMPO.[3]
References
- Nabyl Merbouh; James M. Bobbitt; Christian Brückner (2004). "Preparation of Tetramethylpiperdine-1-oxoammonlum Salts and Their Use as Oxidants in Organic Chemistry. A Review". Organic Preparations and Procedures International. 36: 1-31. doi:10.1080/00304940409355369. S2CID 98117103.
- Sorgi, K. L. "2,2,6,6-Tetramethylpiperidine" in Encyclopedia of Reagents for Organic Synthesis (Ed: L. Paquette) 2004, J. Wiley & Sons, New York. doi:10.1002/047084289X.
- Ciriminna, Rosaria; Pagliaro, Mario (15 January 2010). "Industrial Oxidations with Organocatalyst TEMPO and Its Derivatives". Organic Process Research & Development. 14 (1): 245–251. doi:10.1021/op900059x.