Acivicin
Acivicin is an analog of glutamine. It is an inhibitor of gamma-glutamyl transferase.
 | |
| Names | |
|---|---|
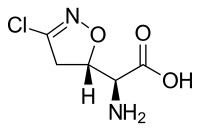
| IUPAC name
(2S)-Amino[(5S)-3-chloro-4,5-dihydro-1,2-oxazol-5-yl]ethanoic acid | |
| Other names
Antibiotic AT 125 | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C5H7ClN2O3 | |
| Molar mass | 178.574 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
It is a fermentation product of Streptomyces sviceus.[1] It interferes with glutamate metabolism and inhibits glutamate dependent synthesis of enzymes, and is thereby potentially helpful in treatment of solid tumors.[2]
After its discovery in 1972, acivicin was studied as an anti-cancer agent, but trials were unsuccessful due to toxicity.[3]
Research
An in vitro study showed that Acivicin at a concentration of 5 μM Acivicin inhibited by 78% the growth of human pancreatic carcinoma cells (MIA PaCa-2) after 72 hours in continuous culture. It was also found that acivicin at a concentration of 450 μM irreversibly inactivated MIA PaCa-2 γ-glutamyl transpeptidase (10 nmol/min/106 cells) with an inactivation half-life of 80 minutes.[1]
Phase I studies
Phase I dose escalating studies conducted in 23 cancer patients administered acivicin with a concomitant 96-h i.v. infusion of a mixture of 16 amino acids showed reversible, dose-limiting CNS toxicity, characterized by lethargy, confusion and decreased mental status.
References
- Allen, L.; Meck, R.; Yunis, A. (1980). "The Inhibition of γ-Glutamyl Transpeptidase from Human Pancreatic Carcinoma Cells by (αS,5S)-α-Amino-3-chloro-4,5-dihydro-5-isoxazoleacetic Acid (AT-125; NSC-163501)". Research Communications in Chemical Pathology and Pharmacology. 27 (1): 175–182. PMID 6102405.
- Hidalgo, M.; Rodriguez, G.; Kuhn, J. G.; Brown, T.; Weiss, G.; MacGovren, J. P.; von Hoff, D. D.; Rowinsky, E. K. (1998). "A Phase I and Pharmacological Study of the Glutamine Antagonist Acivicin with the Amino Acid Solution Aminosyn in Patients with Advanced Solid Malignancies". Clinical Cancer Research. 4 (11): 2763–2770. PMID 9829740.
- Kreuzer, Johannes; Bach, Nina C.; Forler, Daniel; Sieber, Stephan A. (2015). "Target discovery of acivicin in cancer cells elucidates its mechanism of growth inhibition". Chemical Science. 6 (1): 237–245. doi:10.1039/C4SC02339K. PMC 4285139. PMID 25580214.
External links
- Obrador, E.; Carretero, J.; Ortega, A.; Medina, I.; Rodilla, V.; Pellicer, J. A.; Estrela, J. M. (2002). "γ-Glutamyl Transpeptidase Overexpression Increases Metastatic Growth of B16 Melanoma Cells in the Mouse Liver". Hepatology. 35 (1): 74–81. doi:10.1053/jhep.2002.30277. PMID 11786961.
- Schmees, C.; Prinz, C.; Treptau, T.; Rad, R.; Hengst, L.; Voland, P.; Bauer, S.; Brenner, L.; Schmid, R. M.; Gerhard, M. (2007). "Inhibition of T-Cell Proliferation by Helicobacter pylori γ-Glutamyl Transpeptidase". Gastroenterology. 132 (5): 1820–1833. doi:10.1053/j.gastro.2007.02.031. PMID 17484877.