Allylpalladium chloride dimer
Allylpalladium(II) chloride dimer (APC) is a chemical compound with the formula [(η3-C3H5)PdCl]2. This yellow air-stable compound is an important catalyst used in organic synthesis.[2] It is one of the most widely used transition metal allyl complexes.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Allylpalladium(II) chloride dimer | |
| Other names
Allylpalladium chloride dimer bis(allyl)di-μ-chloro-dipalladium(II) APC | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.031.423 |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H10Cl2Pd2 | |
| Molar mass | 365.85 g/mol |
| Appearance | Pale yellow, crystalline solid |
| Melting point | decomp at 155-156 °C |
| Insoluble | |
| Solubility in other solvents | Chloroform benzene acetone methanol |
| Structure[1] | |
| monoclinic | |
| P21/n, No. 14 | |
Formula units (Z) |
2 |
| Hazards | |
| GHS labelling: | |
 | |
| Warning | |
| H302, H312, H315, H319, H332, H335 | |
| P261, P264, P270, P271, P280, P301+P312, P302+P352, P304+P312, P304+P340, P305+P351+P338, P312, P321, P322, P330, P332+P313, P337+P313, P362, P363, P403+P233, P405, P501 | |
| Safety data sheet (SDS) | http://www.colonialmetals.com/pdf/5048.pdf |
| Related compounds | |
Related compounds |
(η3-allyl)(η5 – cyclopentadienyl)palladium(II) di-μ-chlorobis(crotyl)dipalladium |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Structure
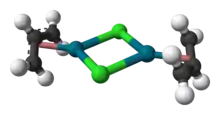
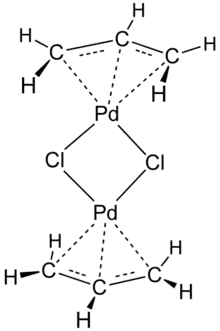
The compound has a dimeric structure that is centrosymmetric. Each allyl group lies in a plane at an angle of about 111.5° to the square formed by the palladium and carbon atoms, and the Pd–C distances are all equal. Its unit cell is monoclinic.[1]
Synthesis
The compound is prepared by purging carbon monoxide through a methanolic aqueous solution of sodium tetrachloropalladate (prepared from palladium(II) chloride and sodium chloride), and allyl chloride.[2]
- 2 Na2PdCl4 + 2 CH2=CHCH2Cl + 2 CO + 2 H2O → [(η3-C3H5)PdCl]2 + 4 NaCl + 2 CO2 + 4 HCl
Another method is the reaction of propene with palladium(II) trifluoroacetate, followed by ion exchange with chloride:[3]
- 2 (CF3COO)2Pd + 2 CH2=CHCH3 → [(η3-C3H5)Pd(CF3COO)]2
- [(η3-C3H5)Pd(CF3COO)]2 + 2 Cl− → [(η3-C3H5)PdCl]2 + 2 CF3COO−
Reactions
APC reacts with sources of cyclopentadienyl anion to give the corresponding 18e− complex cyclopentadienyl allyl palladium:
- [(η3-C3H5)PdCl]2 + 2 NaC5H5 → 2 [(η5-C5H5)Pd(η3-C3H5)] + 2 NaCl
The dimer reacts with a variety of Lewis bases (:B) to form adducts (η3-C3H5)PdCl:B. Its reaction with pyridine and the corresponding enthalpy are:
- 1/2 [(η3-C3H5)PdCl]2 + :NC5H5 → (η3-C3H5)PdCl:NC5H5 ΔH=−30.1 kJ.mol−1
This enthalpy corresponds to the enthalpy change for a reaction forming one mole of the product, (η3-C3H5)PdCl:NC5H5, from the acid dimer. The dissociation energy for the Pd dimer, which is an energy contribution prior to reaction with the donor,
- [(η3-C3H5)PdCl]2 → 2 (η3-C3H5)PdCl
has been determined by the ECW model to be 28 kJ.mol−1.
APC catalyzes many organic reactions, such as cross-coupling, nucleophilic addition to dienes, and decomposition of diazo compounds to reactive carbenes. It is also a useful precursor of other Pd catalysts.[3]
References
- Smith, A. E. (1965). "The structure of the allylpalladium chloride complex (C3H5PdCl)2 at –140°C". Acta Crystallographica. 18 (3): 331–340. doi:10.1107/S0365110X65000774. ISSN 0365-110X.
- Tatsuno, Y.; Yoshida, T.; Otsuka, S. "(η3-allyl)palladium(II) Complexes" Inorganic Syntheses, 1990, volume 28, pages 342-345. ISBN 0-471-52619-3
- Godleski, Stephen A.; Michelet, Véronique; Genêt, Jean-Pierre (2006), "Bis(allyl)di-μ-chlorodipalladium", Encyclopedia of Reagents for Organic Synthesis, Chichester, UK: John Wiley & Sons, Ltd, doi:10.1002/047084289x.rb098s.pub2, ISBN 978-0-471-93623-7, retrieved 2020-09-06