Henri Becquerel
Antoine Henri Becquerel (/ˌbɛkəˈrɛl/;[3] French pronunciation: [ɑ̃twan ɑ̃ʁi bɛkʁɛl]; 15 December 1852 – 25 August 1908) was a French engineer, physicist, Nobel laureate, and the first person to discover radioactivity. For work in this field he, along with Marie Skłodowska-Curie and Pierre Curie,[4] received the 1903 Nobel Prize in Physics. The SI unit for radioactivity, the becquerel (Bq), is named after him.
Henri Becquerel | |
|---|---|
 Portrait by Nadar, c. 1905 | |
| Born | Antoine Henri Becquerel 15 December 1852 Paris, France |
| Died | 25 August 1908 (aged 55) Le Croisic, Brittany, France |
| Alma mater | |
| Known for | Discovery of radioactivity |
| Children | Jean |
| Parent |
|
| Relatives | Antoine César Becquerel (grandfather) |
| Awards |
|
| Scientific career | |
| Fields | Physics, chemistry |
| Institutions |
|
| Thesis | Recherches sur l'absorption de la lumière |
| Doctoral advisor | Charles Friedel[2] |
| Doctoral students | Marie Skłodowska-Curie |
| Signature | |
Biography
Early life
Becquerel was born in Paris, France, into a wealthy family which produced four generations of notable physicists, including Becquerel's grandfather (Antoine César Becquerel), father (Alexandre-Edmond Becquerel), and son (Jean Becquerel).[5] Henri started off his education by attending the Lycée Louis-le-Grand school, a prep school in Paris.[5] He studied engineering at the École Polytechnique and the École des Ponts et Chaussées.[6]
Career
In Becquerel's early career, he became the third in his family to occupy the physics chair at the Muséum National d'Histoire Naturelle in 1892. Later on in 1894, Becquerel became chief engineer in the Department of Bridges and Highways before he started with his early experiments. Becquerel's earliest works centered on the subject of his doctoral thesis: the plane polarization of light, with the phenomenon of phosphorescence and absorption of light by crystals.[7] Early in his career, Becquerel also studied the Earth's magnetic fields.[7] In 1895, he was appointed as a professor at the École Polytechnique.[8]
Becquerel's discovery of spontaneous radioactivity is a famous example of serendipity, of how chance favors the prepared mind. Becquerel had long been interested in phosphorescence, the emission of light of one color following a body's exposure to light of another color. In early 1896, there was a wave of excitement following Wilhelm Conrad Röntgen's discovery of X-rays on 5 January. During the experiment, Röntgen "found that the Crookes tubes he had been using to study cathode rays emitted a new kind of invisible ray that was capable of penetrating through black paper".[9] Learning of Röntgen's discovery from earlier that year during a meeting of the French Academy of Sciences caused Becquerel to be interested, and soon "began looking for a connection between the phosphorescence he had already been investigating and the newly discovered x-rays"[9] of Röntgen, and thought that phosphorescent materials, such as some uranium salts, might emit penetrating X-ray-like radiation when illuminated by bright sunlight.`
By May 1896, after other experiments involving non-phosphorescent uranium salts, he arrived at the correct explanation, namely that the penetrating radiation came from the uranium itself, without any need for excitation by an external energy source.[10] There followed a period of intense research into radioactivity, including the determination that the element thorium is also radioactive and the discovery of additional radioactive elements polonium and radium by Marie Skłodowska-Curie and her husband Pierre Curie. The intensive research of radioactivity led to Becquerel publishing seven papers on the subject in 1896.[6] Becquerel's other experiments allowed him to research more into radioactivity and figure out different aspects of the magnetic field when radiation is introduced into the magnetic field. "When different radioactive substances were put in the magnetic field, they deflected in different directions or not at all, showing that there were three classes of radioactivity: negative, positive, and electrically neutral."[11]
As often happens in science, radioactivity came close to being discovered nearly four decades earlier in 1857, when Abel Niépce de Saint-Victor, who was investigating photography under Michel Eugène Chevreul, observed that uranium salts emitted radiation that could darken photographic emulsions.[12][13] By 1861, Niepce de Saint-Victor realized that uranium salts produce "a radiation that is invisible to our eyes".[14] Niepce de Saint-Victor knew Edmond Becquerel, Henri Becquerel's father. In 1868, Edmond Becquerel published a book, La lumière: ses causes et ses effets (Light: Its causes and its effects). On page 50 of volume 2, Edmond noted that Niepce de Saint-Victor had observed that some objects that had been exposed to sunlight could expose photographic plates even in the dark.[15] Niepce further noted that on the one hand, the effect was diminished if an obstruction were placed between a photographic plate and the object that had been exposed to the sun, but " … d'un autre côté, l'augmentation d'effet quand la surface insolée est couverte de substances facilement altérables à la lumière, comme le nitrate d'urane … " ( ... on the other hand, the increase in the effect when the surface exposed to the sun is covered with substances that are easily altered by light, such as uranium nitrate ... ).[15]
Experiments

Describing them to the French Academy of Sciences on 27 February 1896, he said:
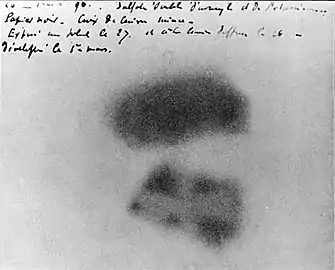
One wraps a Lumière photographic plate with a bromide emulsion in two sheets of very thick black paper, such that the plate does not become clouded upon being exposed to the sun for a day. One places on the sheet of paper, on the outside, a slab of the phosphorescent substance, and one exposes the whole to the sun for several hours. When one then develops the photographic plate, one recognizes that the silhouette of the phosphorescent substance appears in black on the negative. If one places between the phosphorescent substance and the paper a piece of money or a metal screen pierced with a cut-out design, one sees the image of these objects appear on the negative ... One must conclude from these experiments that the phosphorescent substance in question emits rays which pass through the opaque paper and reduce silver salts.[16][17]
But further experiments led him to doubt and then abandon this hypothesis. On 2 March 1896 he reported:
I will insist particularly upon the following fact, which seems to me quite important and beyond the phenomena which one could expect to observe: The same crystalline crusts [of potassium uranyl sulfate], arranged the same way with respect to the photographic plates, in the same conditions and through the same screens, but sheltered from the excitation of incident rays and kept in darkness, still produce the same photographic images. Here is how I was led to make this observation: among the preceding experiments, some had been prepared on Wednesday the 26th and Thursday the 27th of February, and since the sun was out only intermittently on these days, I kept the apparatuses prepared and returned the cases to the darkness of a bureau drawer, leaving in place the crusts of the uranium salt. Since the sun did not come out in the following days, I developed the photographic plates on the 1st of March, expecting to find the images very weak. Instead the silhouettes appeared with great intensity ... One hypothesis which presents itself to the mind naturally enough would be to suppose that these rays, whose effects have a great similarity to the effects produced by the rays studied by M. Lenard and M. Röntgen, are invisible rays emitted by phosphorescence and persisting infinitely longer than the duration of the luminous rays emitted by these bodies. However, the present experiments, without being contrary to this hypothesis, do not warrant this conclusion. I hope that the experiments which I am pursuing at the moment will be able to bring some clarification to this new class of phenomena.[18][19]
Late career
Later in his life in 1900, Becquerel measured the properties of Beta Particles, and he realized that they had the same measurements as high speed electrons leaving the nucleus.[6][20] In 1901 Becquerel made the discovery that radioactivity could be used for medicine. Henri made this discovery when he left a piece of radium in his vest pocket and noticed that he had been burnt by it. This discovery led to the development of radiotherapy which is now used to treat cancer.[6] Becquerel did not survive much longer after his discovery of radioactivity and died on 25 August 1908, at the age of 55, in Le Croisic, France.[7] His death was from unknown causes, but was reported that "he had developed serious burns on his skin, likely from the handling of radioactive materials."[21]
Honors and awards
In 1889, Becquerel became a member of the Académie des Sciences.[6] In 1900, Becquerel won the Rumford Medal for his discovery of the radioactivity of uranium and he awarded the title of an Officer of the Legion of Honour.[22][7] The Berlin-Brandenburg Academy of Sciences and Humanities awarded him the Helmholtz Medal in 1901.[23] In 1902, he was elected as a member of the American Philosophical Society.[24] In 1903, Henri shared a Nobel Prize in Physics with Pierre Curie and Marie Curie for the discovery of spontaneous radioactivity.[7] In 1905, he was awarded the Barnard Medal by the U.S. National Academy of Sciences.[25] In 1906, Henri was elected Vice Chairman of the academy, and in 1908, the year of his death, Becquerel was elected Permanent Secretary of the Académie des Sciences.[26] During his lifetime, Becquerel was honored with membership into the Accademia dei Lincei and the Royal Academy of Berlin.[7] Becquerel was elected a Foreign Member of the Royal Society (ForMemRS) in 1908.[1] Becquerel has been honored with being the namesake of many different scientific discoveries. The SI unit for radioactivity, the becquerel (Bq), is named after him.[27]
There is a crater named Becquerel on the Moon and also a crater named Becquerel on Mars.[28][29] The uranium-based mineral becquerelite was named after Henri.[30] Minor planet 6914 Becquerel is named in his honor.[31]
See also
- A. E. Becquerel (his father)
- Antoine César Becquerel (his grandfather)
- Jean Becquerel (his son)
- Becquerel (SI unit)
- Becquerelite (mineral)
References
- "Fellows of the Royal Society". London: Royal Society. Archived from the original on 16 March 2015.
- "Becquerel, Henri, 1852–1908". history.aip.org. Retrieved 17 April 2022.
- "Becquerel". Random House Webster's Unabridged Dictionary.
- "The Discovery of Radioactivity". Berkeley Lab. Archived from the original on 15 June 2020. Retrieved 28 May 2012.
- Henri Becquerel. [S.l.]: Great Neck Publishing. 2006. ISBN 9781429816434. OCLC 1002022209.
- "Henri Becquerel". Nobel Prize. 1903. Retrieved 15 July 2019.
- Henri Becquerel – Biographical. Nobelprize.org.
- Atomic Heritage Foundation. "Henri Becquerel - Nuclear Museum". Nuclear Museum. Retrieved 10 July 2023.
- Tretkoff, Ernie (March 2008). "American Physical Society".
- "This month in physics history March 1, 1896 Henri Becquerel discovers radioactivity". APS News. 17:3. March 2008.
- "The Discovery of Radioactivity". Guide to the Nuclear Wallchart. 9 August 2000.
- Niepce de Saint-Victor (1857) "Mémoire sur une nouvelle action de la lumière" (On a new action of light), Comptes rendus ... , vol. 45, pages 811–815.
- Niepce de Saint-Victor (1858) "Deuxième mémoire sur une nouvelle action de la lumière" (Second memoir on a new action of light), Comptes rendus ... , vol. 46, pages 448–452.
- Frog, Max. "The man who Discover the world". Retrieved 13 April 2018.
- Edmond Becquerel, La lumière: ses causes et ses effets, vol. 2 (Paris, France: F. Didot, 1868), page 50.
- Henri Becquerel (1896). "Sur les radiations émises par phosphorescence". Comptes Rendus. 122: 420–421.
- Comptes Rendus 122: 420 (1896), translated by Carmen Giunta. Accessed 02 March 2019.
- Henri Becquerel (1896). "Sur les radiations invisibles émises par les corps phosphorescents". Comptes Rendus. 122: 501–503.
- Comptes Rendus 122: 501–503 (1896), translated by Carmen Giunta. Accessed 02 March 2019.
- "Henri Becquerel - Biography, Facts and Pictures". www.famousscientists.org. Retrieved 6 March 2018.
- "Benchmarks: Henri Becquerel discovers radioactivity on February 26, 1896". EARTH Magazine. 5 January 2012. Retrieved 13 April 2018.
- "Rumford Medal". royalsociety.org. Retrieved 12 March 2018.
- "Henri Becquerel". www.nndb.com. Retrieved 25 April 2018.
- "APS Member History". search.amphilsoc.org. Retrieved 19 May 2021.
- "Becquerel, Henri, 1852-1908". history.aip.org. Retrieved 12 March 2018.
- Sekiya, Masaru; Yamasaki, Michio (January 2015). "Antoine Henri Becquerel (1852–1908): a scientist who endeavored to discover natural radioactivity". Radiological Physics and Technology. 8 (1): 1–3. doi:10.1007/s12194-014-0292-z. PMID 25318898 – via Springer Link.
- "BIPM - Becquerel". www.bipm.org. Archived from the original on 25 May 2019. Retrieved 13 April 2018.
- "Planetary Names: Crater, craters: Becquerel on Moon". planetarynames.wr.usgs.gov. Archived from the original on 27 March 2018. Retrieved 13 April 2018.
- "Planetary Names: Crater, craters: Becquerel on Mars". planetarynames.wr.usgs.gov. Archived from the original on 14 April 2018. Retrieved 13 April 2018.
- "Becquerelite: Becquerelite mineral information and data". www.mindat.org. Retrieved 13 April 2018.
- "(6914) Becquerel". Dictionary of Minor Planet Names. Springer. 2003. p. 565. doi:10.1007/978-3-540-29925-7_6180. ISBN 978-3-540-29925-7.
External links
- Henri Becquerel on Nobelprize.org including the Nobel Lecture, "On Radioactivity, a New Property of Matter", 11 December 1903
- Becquerel short biography Archived 7 June 2011 at the Wayback Machine and the use of his name as a unit of measure in the SI
- Annotated bibliography for Henri Becquerel from the Alsos Digital Library for Nuclear Issues
- Henri Becquerel, SI-derived unit of radioactivity
- "Henri Becquerel: The Discovery of Radioactivity", Becquerel's 1896 articles online and analyzed on BibNum [click 'à télécharger' for English version].
- Chisholm, Hugh, ed. (1911). . Encyclopædia Britannica. Vol. 3 (11th ed.). Cambridge University Press.
- "Episode 4 - Henri Becquerel". École polytechnique. 30 January 2019. Archived from the original on 11 December 2021 – via YouTube.