Ariadne (drug)
Ariadne (also known as 4C-D, 4C-DOM, α-Et-2C-D, BL-3912, or dimoxamine) is a little-known psychoactive drug. It is a homologue of the psychedelics 2C-D and DOM. Ariadne was first synthesized by Alexander Shulgin. In his 1991 book PiHKAL, Shulgin reported testing Ariadne up to a dose of 32 mg, and reported that it produced "the alert of a psychedelic, with none of the rest of the package".[1] Very little published data exists about the human pharmacology of Ariadne apart from Shulgin's limited testing; unpublished human trials reportedly observed some psychoactive effects, but no hallucinations.[2][3]
 | |
| Names | |
|---|---|
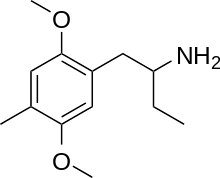
| Preferred IUPAC name
1-(2,5-Dimethoxy-4-methylphenyl)butan-2-amine | |
| Other names
4-Methyl-2,5-dimethoxy-alpha-ethylphenethylamine 4-Methyl-2,5-dimethoxybutanamine | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C13H21NO2 | |
| Molar mass | 223.316 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Shulgin reported that the drug was tested by Bristol Laboratories as an antidepressant, in an anecdote where he was explaining how human testing is invaluable (compared to animal testing) on drugs that change the state of the mind. He said, "Before they launched into a full multi-clinic study to determine whether it's going to be worth the animal studies or not, every person on the board of directors took it."[4]
In animal studies, Ariadne was shown to produce stimulus generalization in rats trained to respond to MDMA[5] or LSD.[2]
References
- Shulgin, Alexander T.; Shulgin, Ann (1991). PiHKAL: a chemical love story (1st ed.). Berkeley, CA: Transform Press. ISBN 9780963009609. OCLC 25627628.
- Winter, J. C. (1980-05-01). "Effects of the phenethylamine derivatives, BL-3912, fenfluramine, and Sch-12679, in rats trained with LSD as a discriminative stimulus". Psychopharmacology. 68 (2): 159–162. doi:10.1007/BF00432134. ISSN 1432-2072. PMID 6776559. S2CID 12221170.
- Cunningham, Michael J.; Bock, Hailey A.; Serrano, Inis C.; Bechand, Benjamin; Vidyadhara, D. J.; Bonniwell, Emma M.; Lankri, David; Duggan, Priscilla; Nazarova, Antonina L.; Cao, Andrew B.; Calkins, Maggie M.; Khirsariya, Prashant; Hwu, Christopher; Katritch, Vsevolod; Chandra, Sreeganga S. (2022-12-15). "Pharmacological Mechanism of the Non-hallucinogenic 5-HT2A Agonist Ariadne and Analogs". ACS Chemical Neuroscience. 14 (1): 119–135. doi:10.1021/acschemneuro.2c00597. ISSN 1948-7193. PMC 10147382. PMID 36521179.
- Alexander Shulgin (2021). The Nature of Drugs. Berkeley, California: Transform Press. pp. 299–300. ISBN 9780999547212.
- Glennon RA (1993). "MDMA-like stimulus effects of alpha-ethyltryptamine and the alpha-ethyl homolog of DOM". Pharmacol Biochem Behav. 46 (2): 459–462. doi:10.1016/0091-3057(93)90379-8. PMID 7903460. S2CID 54356633.