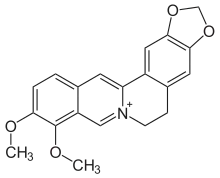
Berberine
Berberine is a quaternary ammonium salt from the protoberberine group of benzylisoquinoline alkaloids
 | |
 | |
| Names | |
|---|---|
| IUPAC name
9,10-Dimethoxy-7,8,13,13a-tetradehydro-2′H-[1,3]dioxolo[4′,5′:2,3]berbin-7-ium | |
| Systematic IUPAC name
9,10-Dimethoxy-5,6-dihydro-2H-7λ5-[1,3]dioxolo[4,5-g]isoquinolino[3,2-a]isoquinolin-7-ylium[1] | |
| Other names | |
| Identifiers | |
3D model (JSmol) |
|
| 3570374 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.016.572 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C20H18NO4+ | |
| Molar mass | 336.366 g·mol−1 |
| Appearance | Yellow solid |
| Melting point | 145 °C (293 °F; 418 K)[3] |
| Slowly soluble[3] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Due to its yellow color, Berberis were used to dye wool, leather, and wood.[4] Under ultraviolet light, berberine shows a strong yellow fluorescence,[5] making it useful in histology for staining heparin in mast cells.[6] As a natural dye, berberine has a color index of 75160.
Research
There is poor-quality evidence that berberine might help with various conditions including metabolic diseases, inflammatory markers, liver and kidney dysfunction, and gastrointestinal disorders.[7] A 2023 review study said that berberine can provide a modest positive impact on lipid concentrations.[8]
Berberine weakly inhibits the CYP2D6 and CYP3A4 enzymes which are involved in metabolism of endogenous substances and xenobiotics, including many prescription drugs.[9]
Biological sources
Berberine is found in such plants as Berberis vulgaris (barberry), Berberis petiolaris, Berberis aristata (tree turmeric), Berberis thunbergii, Mahonia aquifolium (Oregon grape), Hydrastis canadensis (goldenseal), Xanthorhiza simplicissima (yellowroot), Phellodendron amurense (Amur cork tree),[10][11] Coptis chinensis (Chinese goldthread), Tinospora cordifolia, Argemone mexicana (prickly poppy), and Eschscholzia californica (Californian poppy). Berberine is usually found in the roots, rhizomes, stems, and bark.[12]
Biosynthesis

The alkaloid berberine has a tetracyclic skeleton derived from a benzyltetrahydroisoquinoline system with the incorporation of an extra carbon atom as a bridge. Formation of the berberine bridge is rationalized as an oxidative process in which the N-methyl group, supplied by S-adenosyl methionine (SAM), is oxidized to an iminium ion, and a cyclization to the aromatic ring occurs by virtue of the phenolic group.[13]
Reticuline is the immediate precursor of protoberberine alkaloids in plants.[14] Berberine is an alkaloid derived from tyrosine. L-DOPA and 4-hydroxypyruvic acid both come from L-tyrosine. Although two tyrosine molecules are used in the biosynthetic pathway, only the phenethylamine fragment of the tetrahydroisoquinoline ring system is formed via DOPA, the remaining carbon atoms come from tyrosine via 4-hydroxyphenylacetaldehyde. L-DOPA loses carbon dioxide to form dopamine 1. Likewise, 4-hydroxypyruvic acid also loses carbon dioxide to form 4-hydroxyphenylacetaldehyde 2. Dopamine 1 then reacts with 4-hydroxy-phenylacetaldehyde 2 to form (S)-norcoclaurine 3 in a reaction similar to the Mannich reaction. After oxidation and methylation by SAM, (S)-reticuline 4 is formed. (S)-reticuline serves as a pivotal intermediate to other alkaloids. Oxidation of the tertiary amine then occurs and an iminium ion is formed 5. In a Mannich-like reaction the ortho position to the phenol is nucleophilic, and electrons are pushed to form 6. Product 6 then undergoes keto–enol tautomerism to form (S)-scoulerine, which is then methylated by SAM to form (S)-tetrahydrocolumbamine 7. Product 7 is then oxidized to form the methylenedioxy ring from the ortho-methoxyphenol, via an O2-, NADPH- and cytochrome P450-dependent enzyme, giving (S)-canadine 8. (S)-canadine is then oxidized to give the quaternary isoquinolinium system of berberine. This happens in two separate oxidation steps, both requiring molecular oxygen, with H2O2 and H2O produced in the successive processes.[15]
Culture and society
Berberine is classified as a traditional Chinese medicine.[16]
References
- IUPAC Chemical Nomenclature and Structure Representation Division (2013). "P-73.3.1". In Favre, Henri A.; Powell, Warren H. (eds.). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. IUPAC–RSC. ISBN 978-0-85404-182-4.
- The Merck Index, 14th ed., 1154. Berberine
- The Merck Index, 10th Ed. (1983), p.165, Rahway: Merck & Co.
- Gulrajani ML (2001). "Present status of natural dyes". Indian Journal of Fibre & Textile Research. 26: 191–201 – via NISCAIR Online Periodicals Repository.
- Weiß D (2008). "Fluoreszenzfarbstoffe in der Natur" (in German). Retrieved 17 July 2009.
- "B3251 Berberine chloride form". Sigma-Aldrich. 2013. Retrieved 2 Aug 2013.
- Li Z, Wang Y, Xu Q, Ma J, Li X, Yan J, Tian Y, Wen Y, Chen T (May 2023). "Berberine and health outcomes: An umbrella review". Phytother Res. 37 (5): 2051–2066. doi:10.1002/ptr.7806. PMID 36999891.
- Hernandez AV, Hwang J, Nasreen I, Sicignano D, Pasupuleti V, Snow-Caroti K; et al. (2023). "Impact of Berberine or Berberine Combination Products on Lipoprotein, Triglyceride and Biological Safety Marker Concentrations in Patients with Hyperlipidemia: A Systematic Review and Meta-Analysis". J Diet Suppl: 1–18. doi:10.1080/19390211.2023.2212762. PMID 37183391.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Hermann R, von Richter O (September 2012). "Clinical evidence of herbal drugs as perpetrators of pharmacokinetic drug interactions". Planta Medica. 78 (13): 1458–77. doi:10.1055/s-0032-1315117. PMID 22855269.
- Zhang Q, Cai L, Zhong G, Luo W (2010). "Simultaneous determination of jatrorrhizine, palmatine, berberine, and obacunone in Phellodendri Amurensis Cortex by RP-HPLC". Zhongguo Zhong Yao Za Zhi = Zhongguo Zhongyao Zazhi = China Journal of Chinese Materia Medica. 35 (16): 2061–4. doi:10.4268/cjcmm20101603 (inactive 2023-08-28). PMID 21046728.
{{cite journal}}: CS1 maint: DOI inactive as of August 2023 (link) - Cicero, Arrigo F. G.; Baggioni, Alessandra (2016). "Berberine and Its Role in Chronic Disease". Advances in Experimental Medicine and Biology. Vol. 928. Cham: Springer International Publishing. pp. 27–45. doi:10.1007/978-3-319-41334-1_2. ISBN 978-3-319-41332-7. ISSN 0065-2598. PMID 27671811.
- "Berberine". PubChem, National Library of Medicine, US National Institutes of Health. March 9, 2020. Retrieved March 10, 2020.
- Dewick P (2009). Medicinal Natural Products: A Biosynthetic Approach (3rd ed.). West Sussex, England: Wiley. p. 357. ISBN 978-0-471-49641-0.
- Park SU, Facchini PJ (June 2000). "Agrobacterium rhizogenes-mediated transformation of opium poppy, Papaver somniferum l., and California poppy, Eschscholzia californica cham., root cultures". Journal of Experimental Botany. 51 (347): 1005–16. doi:10.1093/jexbot/51.347.1005. PMID 10948228.
- Dewick P (2009). Medicinal Natural Products: A Biosynthetic Approach (3rd ed.). West Sussex, England: Wiley. p. 358. ISBN 978-0-471-49641-0.
- Lin J, Cai Q, Liang B, Wu L, Zhuang Y, He Y, Lin W (January 2019). "Berberine, a Traditional Chinese Medicine, Reduces Inflammation in Adipose Tissue, Polarizes M2 Macrophages, and Increases Energy Expenditure in Mice Fed a High-Fat Diet". Med Sci Monit. 25: 87–97. doi:10.12659/MSM.911849. PMC 6330261. PMID 30606998.