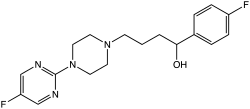
BMY-14802
BMY-14802, also known as BMS-181100, is a drug with antipsychotic effects which acts as both a sigma receptor antagonist and a 5-HT1A receptor agonist.[1][2][3][4] It also has affinity for the 5-HT2 and D4 receptors.[5] The drug reached phase III clinical trials for the treatment of psychosis but was never marketed.[6]
 | |
| Clinical data | |
|---|---|
| Other names | BMS-181100; MJ-14802 |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider |
|
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C18H22F2N4O |
| Molar mass | 348.398 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
See also
- Enciprazine
- Ensaculin
- Mafoprazine
- Azaperone
- Fluanisone
References
- Taylor DP, Eison MS, Moon SL, Schlemmer RF, Shukla UA, VanderMaelen CP, Yocca FD, Gallant DJ, Behling SH, Boissard CG (1993). "A role for sigma binding in the antipsychotic profile of BMY 14802?". NIDA Research Monograph. 133: 125–57. PMID 8232511.
- Vanecek SA, Essman WD, Taylor DP, Woods JH (January 1998). "Discriminative stimulus characteristics of BMY 14802 in the pigeon". The Journal of Pharmacology and Experimental Therapeutics. 284 (1): 1–9. PMID 9435153.
- Kitanaka J, Kitanaka N, Tatsuta T, Hall FS, Uhl GR, Tanaka K, Nishiyama N, Morita Y, Takemura M (May 2009). "Sigma1 receptor antagonists determine the behavioral pattern of the methamphetamine-induced stereotypy in mice". Psychopharmacology. 203 (4): 781–92. doi:10.1007/s00213-008-1425-z. PMC 3157915. PMID 19052726.
- Paquette MA, Foley K, Brudney EG, Meshul CK, Johnson SW, Berger SP (July 2009). "The sigma-1 antagonist BMY-14802 inhibits L-DOPA-induced abnormal involuntary movements by a WAY-100635-sensitive mechanism". Psychopharmacology. 204 (4): 743–54. doi:10.1007/s00213-009-1505-8. PMC 2845289. PMID 19283364.
- Luis M. Botana; Mabel Loza (20 April 2012). Therapeutic Targets: Modulation, Inhibition, and Activation. John Wiley & Sons. pp. 248–. ISBN 978-1-118-18552-0.
- "BMS 181100 - AdisInsight".
| 5-HT1 |
| ||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-HT2 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT3–7 |
| ||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||
| σ1 |
|
|---|---|
| σ2 |
|
| Unsorted |
|
See also: Receptor/signaling modulators | |
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.