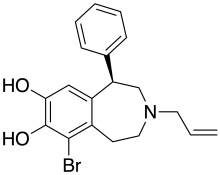
6-Br-APB
6-Br-APB is a synthetic compound that acts as a selective D1 agonist,[1] with the (R)-enantiomer being a potent full agonist, while the (S) enantiomer retains its D1 selectivity but is a weak partial agonist.[2] (R)-6-Br-APB and similar D1-selective full agonists like SKF-81,297 and SKF-82,958 produce characteristic anorectic effects, stereotyped behaviour and self-administration in animals, with a similar but not identical profile to that of dopaminergic stimulants such as amphetamine.[3][4][5]
 | |
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C19H20BrNO2 |
| Molar mass | 374.278 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
References
- Neumeyer JL, et al. (December 1991). "(+/-)-3-Allyl-6-bromo-7,8-dihydroxy-1-phenyl-2,3,4,5-tetrahydro-1H-3- benzazepin, a new high-affinity D1 dopamine receptor ligand: synthesis and structure-activity relationship". Journal of Medicinal Chemistry. 34 (12): 3366–71. doi:10.1021/jm00116a004. PMID 1684995.
- Neumeyer JL, Kula NS, Baldessarini RJ, Baindur N (April 1992). "Stereoisomeric probes for the D1 dopamine receptor: synthesis and characterization of R-(+) and S-(-) enantiomers of 3-allyl-7,8-dihydroxy-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine and its 6-bromo analogue". Journal of Medicinal Chemistry. 35 (8): 1466–71. doi:10.1021/jm00086a016. PMID 1533424.
- Rosenzweig-Lipson S, Hesterberg P, Bergman J (September 1994). "Observational studies of dopamine D1 and D2 agonists in squirrel monkeys". Psychopharmacology. 116 (1): 9–18. doi:10.1007/BF02244865. PMID 7862937. S2CID 24204026.
- Weed MR, Woolverton WL (December 1995). "The reinforcing effects of dopamine D1 receptor agonists in rhesus monkeys". The Journal of Pharmacology and Experimental Therapeutics. 275 (3): 1367–74. PMID 8531104.
- Barrett AC, Miller JR, Dohrmann JM, Caine SB (2004). "Effects of dopamine indirect agonists and selective D1-like and D2-like agonists and antagonists on cocaine self-administration and food maintained responding in rats". Neuropharmacology. 47 (Suppl 1): 256–73. doi:10.1016/j.neuropharm.2004.07.007. PMID 15464142. S2CID 2959198.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.