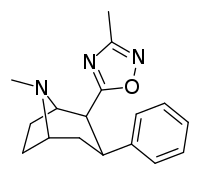
RTI-126
RTI-126 (RTI-4229-126 or (–)-2β-(1,2,4-oxadiazol-5-methyl)-3β-phenyltropane) is a phenyltropane derivative which acts as a potent monoamine reuptake inhibitor and stimulant drug, and has been sold as a designer drug. It is around 5 times more potent than cocaine at inhibiting monoamine reuptake in vitro, but is relatively unselective. It binds to all three monoamine transporters, although still with some selectivity for the dopamine transporter.[1] RTI-126 has a fast onset of effects and short duration of action, and its pharmacological profile in animals is among the closest to cocaine itself out of all the drugs in the RTI series. Its main application in scientific research has been in studies investigating the influence of pharmacokinetics on the abuse potential of stimulant drugs, with its rapid entry into the brain thought to be a key factor in producing its high propensity for development of dependence in animals.[2][3]
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| Chemical and physical data | |
| Formula | C17H21N3O |
| Molar mass | 283.375 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
The structurally related compound (–)-2β-(3-methyl-5-isoxazolyl)nortropane is a potent and selective agonist for nicotinic acetylcholine receptors, with twice the potency of nicotine.[4]
nortropane_structure.png.webp)
See also
References
- Carroll FI, Gray JL, Abraham P, Kuzemko MA, Lewin AH, Boja JW, Kuhar MJ (October 1993). "3-Aryl-2-(3'-substituted-1',2',4'-oxadiazol-5'-yl)tropane analogues of cocaine: affinities at the cocaine binding site at the dopamine, serotonin, and norepinephrine transporters". Journal of Medicinal Chemistry. 36 (20): 2886–90. doi:10.1021/jm00072a007. PMID 8411004.
- Kimmel HL, Carroll FI, Kuhar MJ (December 2001). "Locomotor stimulant effects of novel phenyltropanes in the mouse". Drug and Alcohol Dependence. 65 (1): 25–36. doi:10.1016/S0376-8716(01)00144-2. PMID 11714587.
- Kimmel HL, O'Connor JA, Carroll FI, Howell LL (January 2007). "Faster onset and dopamine transporter selectivity predict stimulant and reinforcing effects of cocaine analogs in squirrel monkeys". Pharmacology, Biochemistry, and Behavior. 86 (1): 45–54. doi:10.1016/j.pbb.2006.12.006. PMC 1850383. PMID 17258302.
- Cheng J, Izenwasser S, Zhang C, Zhang S, Wade D, Trudell ML (April 2004). "Synthesis and nicotinic acetylcholine receptor binding affinities of 2- and 3-isoxazolyl-8-azabicyclo[3.2.1]octanes". Bioorganic & Medicinal Chemistry Letters. 14 (7): 1775–8. doi:10.1016/j.bmcl.2004.01.025. PMID 15026069.