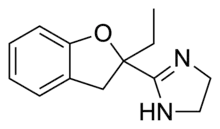
Efaroxan
Efaroxan is an α2-adrenergic receptor antagonist[1] and antagonist of the imidazoline receptor.
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C13H16N2O |
| Molar mass | 216.284 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Synthesis
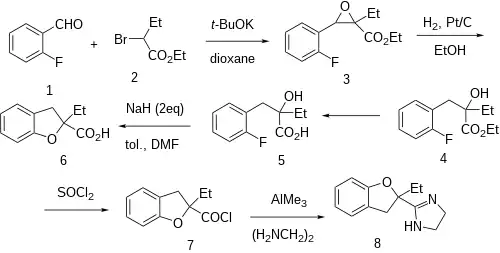
The Darzens reaction between 2-fluorobenzaldehyde [57848-46-1] (1) and Ethyl 2-bromobutyrate [533-68-6] (2) gives ethyl 2-ethyl-3-(2-fluorophenyl)oxirane-2-carboxylate, CID:100942311 (3). A catalytic hydrogenation over Pd/C would give ethyl 2-[(2-fluorophenyl)methyl]-2-hydroxybutanoate, CID:77591056 (4). Saponification of the ester then gives 2-[(2-Fluorophenyl)methyl]-2-hydroxybutanoic acid, CID:53869347 (5). Treatment with 2 molar equivalents of sodium hydride apparently gives 2-Ethyl-2,3-dihydrobenzofuran-2-carboxylic acid [111080-50-3] (6). Treatment of the carboxylic acid with thionyl chloride then gives the acid chloride and subsequent treatment of this with ethylenediamine in the presence of trimethylaluminium completed the synthesis of Efaroxan (8).
See also
References
- Chopin P, Colpaert FC, Marien M (February 1999). "Effects of alpha-2 adrenoceptor agonists and antagonists on circling behavior in rats with unilateral 6-hydroxydopamine lesions of the nigrostriatal pathway". J. Pharmacol. Exp. Ther. 288 (2): 798–804. PMID 9918591.
- Chapleo, Christopher B.; Myers, Peter L.; Butler, Richard C. M.; Davis, John A.; Doxey, John C.; Higgins, Stanley D.; Myers, Malcolm; Roach, Alan G.; Smith, Colin F. C. (1984). ".alpha.-Adrenoceptor reagents. 2. Effects of modification of the 1,4-benzodioxan ring system on .alpha.-adrenoreceptor activity". Journal of Medicinal Chemistry 27 (5): 570–576. doi:10.1021/jm00371a003.
- Mayer, P.; Brunel, P.; Imbert, T. (1999). "A new efficient synthesis of efaroxan". Bioorganic & Medicinal Chemistry Letters 9 (20): 3021–3022. doi:10.1016/S0960-894X(99)00531-4.
- Couture, K.; Gouverneur, V.; Mioskowski, C. (1999). "A new approach to the synthesis of efaroxan". Bioorganic & Medicinal Chemistry Letters 9 (20): 3023–3026. doi:10.1016/S0960-894X(99)00530-2.