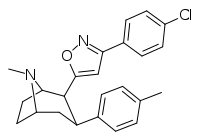
RTI-371
3β-(4-Methylphenyl)-2β-[3-(4-chlorophenyl)isoxazol-5-yl]tropane (RTI-4229-371) is a phenyltropane derived drug which acts as a potent and selective dopamine reuptake inhibitor in vitro, yet unusually for this class of compound, both RTI-371 and the closely related compound RTI-370 failed to produce locomotor stimulation in mice. In addition to this, in drug substitution tests RTI-370 weakly generalized to cocaine whereas RTI-371 did not generalize at all.
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| Chemical and physical data | |
| Formula | C24H25ClN2O |
| Molar mass | 392.93 g·mol−1 |
| 3D model (JSmol) | |
| |
| | |
This phenomenon has also been observed for other dopamine reuptake inhibitors from other classes. It may be caused by lack of BBB penetration, or interactions at alternative receptor sites.[1][2]
References
- Navarro HA, Howard JL, Pollard GT, Carroll FI (April 2009). "Positive allosteric modulation of the human cannabinoid (CB) receptor by RTI-371, a selective inhibitor of the dopamine transporter". British Journal of Pharmacology. 156 (7): 1178–84. doi:10.1111/j.1476-5381.2009.00124.x. PMC 2697692. PMID 19226282.
- Foster MD (2011). Computational study of RTI-371, a positive allosteric modulator of the cannabinoid CB1 receptor (PDF) (MSc thesis). The University of North Carolina at Greensboro.
| 2-Carboxymethyl Esters | |
|---|---|
| (3,4-Disubstituted Phenyl)-tropanes | |
| Arylcarboxy | |
| Carboxyalkyl | |
| Acyl | |
| β,α Stereochemistry | |
| α,β Stereochemistry | |
| Heterocycles: 3-Substituted-isoxazol-5-yl | |
| Heterocycles: 3-Substituted-1,2,4-oxadiazole | |
| N-alkyl | |
| N-replaced (S,O,C) | |
| Irreversible | |
| Nortropanes (N-demethylated) | |
| D1-like |
| ||||||
|---|---|---|---|---|---|---|---|
| D2-like |
| ||||||
| |||||||
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.