Tavapadon
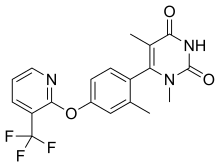
Tavapadon (developmental code names CVL-751, PF-06649751) is a dopamine receptor agonist for the treatment of Parkinson's disease.[1][2][3], under development by Cerevel Therapeutics who acquired Tavapadon from Pfizer in 2018. It acts as a selective partial agonist of the dopamine D1 (Ki = 8.54 nM) and D5 receptors.[2][3] It also shows biased agonism for Gs-coupled signaling.[2] As of July 2021, tavapadon is in phase 3 clinical trials for Parkinson's disease.[1]
 | |
| Clinical data | |
|---|---|
| Other names | CVL-751; PF-6649751; PF-06649751 |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C19H16F3N3O3 |
| Molar mass | 391.350 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
See also
References
- "Tavapadon - Cerevel Therapeutics". Adis Insight. Springer Nature Switzerland AG.
- Cerri S, Blandini F (December 2020). "An update on the use of non-ergot dopamine agonists for the treatment of Parkinson's disease". Expert Opinion on Pharmacotherapy. 21 (18): 2279–2291. doi:10.1080/14656566.2020.1805432. PMID 32804544.
- Hall A, Provins L, Valade A (January 2019). "Novel Strategies To Activate the Dopamine D1 Receptor: Recent Advances in Orthosteric Agonism and Positive Allosteric Modulation". Journal of Medicinal Chemistry. 62 (1): 128–140. doi:10.1021/acs.jmedchem.8b01767. PMID 30525590.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.