Moperone
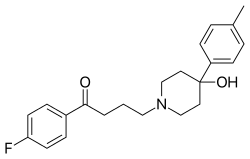
Moperone (Luvatren, since discontinued) is a typical antipsychotic of the butyrophenone class[2] which is marketed in Japan for the treatment of schizophrenia. It is an antagonist for the D2 (Ki 0.7–1.9 nM), D3 (Ki 0.1–1 nM), and 5-HT2A (Ki 52 nM) receptors. It also has a high binding affinity for the sigma receptors.[3][4]
 | |
| Clinical data | |
|---|---|
| Trade names | Luvatren (discontinued) |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.012.625 |
| Chemical and physical data | |
| Formula | C22H26FNO2 |
| Molar mass | 355.453 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
References
- Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-16.
- Gross H, Kaltenbäck E (1969). "The clinical position of moperone among the butyrophenones". Nordisk Psykiatrisk Tidsskrift. Nordic Journal of Psychiatry. 23 (1): 4–9. doi:10.3109/08039486909132154. PMID 5354545.
- Miyamoto S (2010). "Moperone". In Stolerman IP (ed.). Encyclopedia of Psychopharmacology. Berlin, Heidelberg: Springer. p. 798. doi:10.1007/978-3-540-68706-1_1838. ISBN 978-3-540-68706-1. Retrieved 21 March 2022.
- Roth BL, Driscol J. "PDSP Ki Database". Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. Retrieved 21 March 2022.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.