Zicronapine
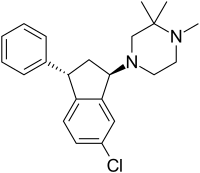
Zicronapine (/zaɪˈkrɒnəpiːn/ zye-KRON-ə-peen, previously known as Lu 31-130) is an atypical antipsychotic medication[1] formerly under development by H. Lundbeck A/S. In phase II studies zicronapine showed statistically significant separation from placebo and convincing efficacy and safety data when compared to olanzapine.[2]
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG |
|
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C22H27ClN2 |
| Molar mass | 354.92 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Zicronapine exhibits monoaminergic activity and has a multi-receptorial profile. In vitro and in vivo it has shown potent antagonistic effects at dopamine D1, D2 and serotonin 5HT2A receptors.[3]
In 2014 Lundbeck removed zicronapine from its development portfolio in favor of pursuing the more promising antipsychotic Lu AF35700 (a prodrug of Lu AF356152).[4]
References
- Citrome L (November 2013). "A review of the pharmacology, efficacy and tolerability of recently approved and upcoming oral antipsychotics: an evidence-based medicine approach". CNS Drugs. 27 (11): 879–911. doi:10.1007/s40263-013-0105-7. PMID 24062193. S2CID 23867019.
- "The clinical phase III programme commenced on zicronapine". January 20, 2011. Archived from the original on 2 February 2017. Retrieved 6 February 2014.
- "Zicronapine shows significant positive data in clinical phase II in the treatment of patients with schizophrenia - planning for continued clinical work". December 18, 2009. Archived from the original on 8 October 2016. Retrieved 6 February 2014.
- "Performance in 2014 positions Lundbeck well for 2015 and beyond" (PDF). February 5, 2015. Archived from the original (PDF) on 18 June 2015. Retrieved 18 June 2015.
External links
 Media related to Zicronapine at Wikimedia Commons
Media related to Zicronapine at Wikimedia Commons
| 5-HT1 |
| ||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-HT2 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT3–7 |
| ||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.