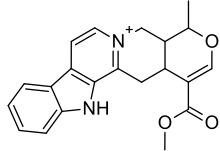
Alstonine
Alstonine is an indoloquinolizidine alkaloid and putative antipsychotic constituent of various plant species including Alstonia boonei, Catharanthus roseus, Picralima nitida, Rauwolfia caffra and Rauwolfia vomitoria.[1] In preclinical studies alstonine attenuates MK-801-induced hyperlocomotion, working memory deficit and social withdrawal.[2] It also possesses anxiolytic-like effects in preclinical studies,[1] attenuates amphetamine-induced lethality and stereotypy as well as apomorphine-induced stereotypy,[1] and attenuates haloperidol-induced catalepsy.[3] These effects appear to be mediated by stimulation of the 5-HT2C receptor.[4] In addition, alstonine, similarly to clozapine, indirectly inhibits the reuptake of glutamate in hippocampal slices.[5] Unlike clozapine however, the effect of which is abolished by the D2 receptor agonist apomorphine, alstonine requires 5-HT2A and 5-HT2C receptors to produce this effect, as it is abolished by antagonists of these receptors. Also unlike clozapine, alstonine lacks pro-convulsant activity in mice.[6]
 | |
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| Chemical and physical data | |
| Formula | C21H21N2O3 |
| Molar mass | 349.410 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
See also
References
- Elisabetsky, E; Costa-Campos, L (March 2006). "The Alkaloid Alstonine: A Review of Its Pharmacological Properties". Evidence-Based Complementary and Alternative Medicine. 3 (1): 39–48. doi:10.1093/ecam/nek011. PMC 1375234. PMID 16550222.
- Linck, VM; Bessa, MM; Herrmann, AP; Iwu, MM; Okunji, CO; Elisabetsky, E (January 2012). "5-HT2A/C receptors mediate the antipsychotic-like effects of alstonine". Progress in Neuro-Psychopharmacology and Biological Psychiatry. 36 (1): 29–33. doi:10.1016/j.pnpbp.2011.08.022. PMID 21925231. S2CID 3236174.
- Linck, VM; Herrmann, AP; Piato, LN; Detanico, BC; Figueir, M; Flório, J; Iwu, MM; Okunji, CO; Leal, MB; Elisabetsky, E (July 2011). "Alstonine as an Antipsychotic: Effects on Brain Amines and Metabolic Changes" (PDF). Evidence-Based Complementary and Alternative Medicine. 2011 (418597): 418597. doi:10.1093/ecam/nep002. PMC 3140158. PMID 19189988.
- Gross, G; Geyer, MA, eds. (2012). Current Antipsychotics. Handbook of Experimental Pharmacology. Vol. 212. Springer Berlin Heidelberg. p. 107. doi:10.1007/978-3-642-25761-2. ISBN 978-3-642-25761-2. ISSN 1865-0325. S2CID 11477428.
- Herrmann, AP; Lunardi, P; Pilz, LK; Tramontina, AC; Linck, VM; Okunji, CO; Gonçalves, CA; Elisabetsky, E (December 2012). "Effects of the putative antipsychotic alstonine on glutamate uptake in acute hippocampal slices". Neurochemistry International. 61 (7): 1144–1150. doi:10.1016/j.neuint.2012.08.006. PMID 22940693. S2CID 14234269.
- Costa-Campos L, Iwu M, Elisabetsky E (August 2004). "Lack of pro-convulsant activity of the antipsychotic alkaloid alstonine". Journal of Ethnopharmacology. 93 (2–3): 307–310. doi:10.1016/j.jep.2004.03.056. PMID 15234769.