Cilansetron
Cilansetron is an experimental drug that is a 5-HT3 antagonist under development by Solvay Pharmaceuticals.[1][2][3]
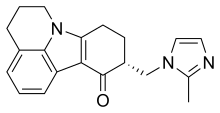 | |
| Clinical data | |
|---|---|
| Other names | Calmactin; KC 9946 |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 87% |
| Metabolism | Hepatic |
| Elimination half-life | 1.6 - 1.9 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C20H21N3O |
| Molar mass | 319.408 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
5-HT3 receptors are responsible for causing many things from nausea to excess bowel movements. In conditions such as irritable bowel syndrome (IBS), the receptors have become faulty or oversensitive. 5-HT3 antagonists work by blocking the nervous and chemical signals from reaching these receptors.
Studies have shown that the drug can improve quality of life in men and women with diarrhea-predominant IBS.[4] Cilansetron is the first 5-HT antagonist specifically designed for IBS that is effective in men as well as women.[4]
In 2005, Solvay received response from the U.S. Food and Drug Administration that cilansertron is not approvable without additional clinical trials;[5][6] further development has been discontinued.[7]
References
- Chey WD, Cash BD (February 2005). "Cilansetron: a new serotonergic agent for the irritable bowel syndrome with diarrhoea". Expert Opinion on Investigational Drugs. 14 (2): 185–93. doi:10.1517/13543784.14.2.185. PMID 15757394. S2CID 8606399.
- Olden KW, Crowell MD (October 2005). "Cilansetron". Drugs of Today. Barcelona, Spain. 41 (10): 661–6. doi:10.1358/dot.2005.41.10.920427. PMID 16389408.
- Stacher G (October 2007). "Cilansetron in the treatment of diarrhea-predominant irritable bowel syndrome?". Expert Review of Gastroenterology & Hepatology. 1 (1): 15–27. doi:10.1586/17474124.1.1.15. PMID 19072430. S2CID 24301639.
- General info on Cilansetron
- "Solvay's cilansetron "not approvable"". PharmaTimes. April 4, 2005. Retrieved August 14, 2019.
- "Calmactin Approval Status". Drugs.com. Retrieved August 14, 2019.
- "Cilansetron". Retrieved August 14, 2019.