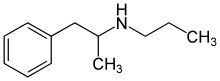
Propylamphetamine
Propylamphetamine is a psychoactive drug and research chemical of the phenethylamine and amphetamine chemical classes which acts as a stimulant. It was first developed in the 1970s, mainly for research into the metabolism of,[1] and as a comparison tool to, other amphetamines.[2] A study in rats found propylamphetamine to be 1/4 as potent as amphetamine.[3]
 | |
 | |
| Clinical data | |
|---|---|
| Other names | N-propyl-1-phenyl-propan-2-amine; N-propylamphetamine |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Hepatic |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider |
|
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.215.934 |
| Chemical and physical data | |
| Formula | C12H19N |
| Molar mass | 177.291 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
References
- Nazarali AJ, Baker GB, Coutts RT, Pasutto FM (1983). "Amphetamine in rat brain after intraperitoneal injection of N-alkylated analogues". Progress in Neuro-Psychopharmacology & Biological Psychiatry. 7 (4–6): 813–6. doi:10.1016/0278-5846(83)90073-8. PMID 6686713. S2CID 35531794.
- Valtier S, Cody JT (October 1995). "Evaluation of internal standards for the analysis of amphetamine and methamphetamine". Journal of Analytical Toxicology. 19 (6): 375–80. doi:10.1093/jat/19.6.375. PMID 8926730.
- Woolverton WL, Shybut G, Johanson CE (December 1980). "Structure-activity relationships among some d-N-alkylated amphetamines". Pharmacology, Biochemistry, and Behavior. 13 (6): 869–76. CiteSeerX 10.1.1.687.9187. doi:10.1016/0091-3057(80)90221-x. PMID 7208552. S2CID 25123820.
| Phenethylamines |
|
|---|---|
| Amphetamines |
|
| Phentermines |
|
| Cathinones |
|
| Phenylisobutylamines | |
| Phenylalkylpyrrolidines | |
| Catecholamines (and close relatives) |
|
| Miscellaneous |
|
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.