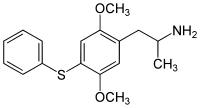
Aleph (psychedelic)
Aleph (also known as DOT or 2,5-dimethoxy-4-methylthioamphetamine) is a psychedelic hallucinogenic drug and a substituted amphetamine of the phenethylamine class of compounds, which can be used as an entheogen. It was first synthesized by Alexander Shulgin, who named it after the first letter of the Hebrew alphabet. In his book PiHKAL, Shulgin lists the dosage range as 5–10 mg, with effects typically lasting for 6 to 8 hours.[1]
 | |
| Names | |
|---|---|
| Preferred IUPAC name
1-[2,5-Dimethoxy-4-(methylsulfanyl)phenyl]propan-2-amine | |
| Other names
2,5-Dimethoxy-4-methylthioamphetamine 1-(4-Methylthio-2,5-dimethoxyphenyl)-2-aminopropane | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C12H19NO2S | |
| Molar mass | 241.35 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Like many other psychedelics, aleph is a partial agonist at the 5-HT2A receptor (EC50 = 10 nM).[2] It has weak MAO-A inhibitory activity with an IC50 of 5.2 μM. For reference, amphetamine has an IC50 of 11 μM and 4-methylthioamphetamine has a value of 0.2 μM.[3] A lower number indicates stronger inhibition.
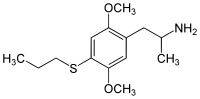
Homologues
Aleph-2
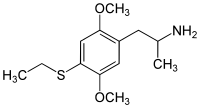
Dosage: 7–12 mg
Duration: 8–16 hours
Effects: Strong visuals
CAS number: 185562-00-9
SMILES: C1(=C(C=C(C(=C1)SCC)OC)CC(C)N)OC
Aleph-4
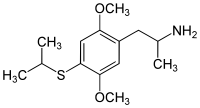
Dosage: 7–12 mg
Duration: 12–20 hours
Effects: "profound and deep learning experiences" - Alexander Shulgin
CAS number: 123643-26-5
SMILES: C1(=C(C=C(C(=C1)SC(C)C)OC)CC(C)N)OC
Legality
In the United States Aleph is a Schedule 1 controlled substance as a positional isomer of 2C-T-4 and 2C-T-7[4]
References
- Shulgin, Alexander; Shulgin, Ann (September 1991). PiHKAL: A Chemical Love Story. Berkeley, California: Transform Press. ISBN 0-9630096-0-5. OCLC 25627628.
- Halberstadt AL, Luethi D, Hoener MC, Trachsel D, Brandt SD, Liechti ME (January 2023). "Use of the head-twitch response to investigate the structure-activity relationships of 4-thio-substituted 2,5-dimethoxyphenylalkylamines". Psychopharmacology. 240 (1): 115–126. doi:10.1007/s00213-022-06279-2. PMC 9816194. PMID 36477925.
- Gallardo-Godoy A, Fierro A, McLean TH, Castillo M, Cassels BK, Reyes-Parada M, Nichols DE (April 2005). "Sulfur-substituted alpha-alkyl phenethylamines as selective and reversible MAO-A inhibitors: biological activities, CoMFA analysis, and active site modeling". Journal of Medicinal Chemistry. 48 (7): 2407–2419. doi:10.1021/jm0493109. PMID 15801832.
- "Lists of: Scheduling Actions Controlled Substances Regulated Chemicals" (PDF). Drug and Chemical Evaluation Section, Diversion Control Division. Drug Enforcement Administration, U.S. Department of Justice. January 2023.
External links
- Aleph Entry in PiHKAL
- Aleph-2 Entry in PiHKAL
- Aleph-4 Entry in PiHKAL
- Aleph-6 Entry in PiHKAL
- Aleph-7 Entry in PiHKAL
| 5-HT1 |
| ||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-HT2 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT3–7 |
| ||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||
| Phenethylamines |
|
|---|---|
| Amphetamines |
|
| Phentermines |
|
| Cathinones |
|
| Phenylisobutylamines | |
| Phenylalkylpyrrolidines | |
| Catecholamines (and close relatives) |
|
| Miscellaneous |
|