JWH-251
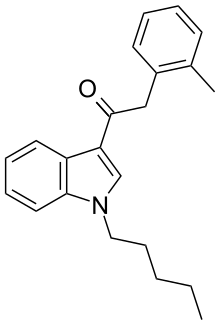
JWH-251 (1-pentyl-3-(2-methylphenylacetyl)indole) is a synthetic cannabinoid from the phenylacetylindole family, which acts as a cannabinoid agonist with about five times selectivity for CB1 with a Ki of 29 nM and 146 nM at CB2. Similar to the related 2'-methoxy compound JWH-250, the 2'-chloro compound JWH-203, and the 2'-bromo compound JWH-249, JWH-251 has a phenylacetyl group in place of the naphthoyl ring used in most aminoalkylindole cannabinoid compounds.[2][3]
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2-(2-Methylphenyl)-1-(1-pentyl-1H-indol-3-yl)ethan-1-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C22H25NO | |
| Molar mass | 319.448 g·mol−1 |
| Pharmacology | |
| Legal status |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
In the United States, all CB1 receptor agonists of the 3-phenylacetylindole class such as JWH-251 are Schedule I Controlled Substances.[4]
References
- Anvisa (2023-07-24). "RDC Nº 804 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 804 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-07-25). Archived from the original on 2023-08-27. Retrieved 2023-08-27.
- Huffman JW, Szklennik PV, Almond A, Bushell K, Selley DE, He H, et al. (September 2005). "1-Pentyl-3-phenylacetylindoles, a new class of cannabimimetic indoles". Bioorganic & Medicinal Chemistry Letters. 15 (18): 4110–3. doi:10.1016/j.bmcl.2005.06.008. PMID 16005223.
- Manera C, Tuccinardi T, Martinelli A (April 2008). "Indoles and related compounds as cannabinoid ligands". Mini Reviews in Medicinal Chemistry. 8 (4): 370–87. doi:10.2174/138955708783955935. PMID 18473928.
- : Schedules of controlled substances
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.