AM-919
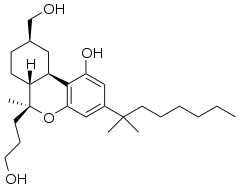
AM-919 (part of the AM cannabinoid series) is an analgesic drug which is a cannabinoid receptor agonist. It is a derivative of HU-210 which has been substituted with a 6β-(3-hydroxypropyl) group. This adds a "southern" aliphatic hydroxyl group to the molecule as seen in the CP-series of nonclassical cannabinoid drugs, and so AM-919 represents a hybrid structure between the classical dibenzopyran and nonclassical cannabinoid families.[1]
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C27H44O4 |
| Molar mass | 432.645 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
AM-919 is somewhat less potent than HU-210 itself, but is still a potent agonist at both CB1 and CB2 with moderate selectivity for CB1, with a Ki of 2.2 nM at CB1 and 3.4 nM at CB2.[2][3]
See also
References
- Pertwee R. Cannabinoids. Handbook of Experimental Pharmacology. Vol. 168. Springer. p. 269. ISBN 3-540-22565-X.
- Tius MA, Hill WA, Zou XL, Busch-Petersen J, Kawakami JK, Fernandez-Garcia MC, et al. (1995). "Classical/non-classical cannabinoid hybrids; stereochemical requirements for the southern hydroxyalkyl chain". Life Sciences. 56 (23–24): 2007–12. doi:10.1016/0024-3205(95)00182-6. PMID 7776825.
- Drake DJ, Jensen RS, Busch-Petersen J, Kawakami JK, Concepcion Fernandez-Garcia M, Fan P, Makriyannis A, Tius MA (September 1998). "Classical/nonclassical hybrid cannabinoids: southern aliphatic chain-functionalized C-6beta methyl, ethyl, and propyl analogues". Journal of Medicinal Chemistry. 41 (19): 3596–608. doi:10.1021/jm960677q. PMID 9733485.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.