CUMYL-FUBINACA
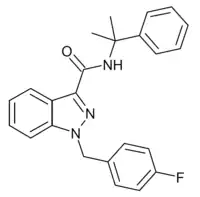
CUMYL-FUBINACA (SGT-149) is an indazole-3-carboxamide based synthetic cannabinoid receptor agonist, with an EC50 of 1.8nM for human CB1 receptors and 23.7nM for human CB2 receptors, giving it around 13x selectivity for CB1.[1][2][3][4] It has been sold online as a designer drug.[5]
 | |
| Legal status | |
|---|---|
| Legal status | |
| Identifiers | |
| |
| PubChem CID | |
| UNII | |
| Chemical and physical data | |
| Formula | C24H22FN3O |
| Molar mass | 387.458 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
References
- WO 2014167530, Bowden MW, Williamson PB, "Cannabinoid compounds", published 11 April 2013
- Longworth M, Banister SD, Boyd R, Kevin RC, Connor M, McGregor IS, Kassiou M (October 2017). "Pharmacology of Cumyl-Carboxamide Synthetic Cannabinoid New Psychoactive Substances (NPS) CUMYL-BICA, CUMYL-PICA, CUMYL-5F-PICA, CUMYL-5F-PINACA, and Their Analogues". ACS Chemical Neuroscience. 8 (10): 2159–2167. doi:10.1021/acschemneuro.7b00267. PMID 28792725.
- Banister SD, Connor M (2018). "The Chemistry and Pharmacology of Synthetic Cannabinoid Receptor Agonist New Psychoactive Substances: Evolution". New Psychoactive Substances. Handbook of Experimental Pharmacology. Vol. 252. pp. 191–226. doi:10.1007/164_2018_144. ISBN 978-3-030-10560-0. PMID 30105473.
- Krishna Kumar K, Shalev-Benami M, Robertson MJ, Hu H, Banister SD, Hollingsworth SA, et al. (January 2019). "Structure of a Signaling Cannabinoid Receptor 1-G Protein Complex". Cell. 176 (3): 448–458.e12. doi:10.1016/j.cell.2018.11.040. PMC 6461403. PMID 30639101.
- Thornton SL, Darracq MA, Gugelmann HM, Armenian P (2019). "Surface internet marketplace presence and availability of NPS sold as research chemicals: a snapshot study". Toxicology Communications. 3 (1): 67–74. doi:10.1080/24734306.2019.1648067. S2CID 202372196.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.