Cannabimovone
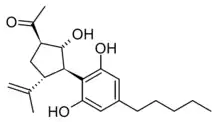
Cannabimovone (CBM) is a phytocannabinoid first isolated from a non-psychoactive strain of Cannabis sativa in 2010, which is thought to be a rearrangement product of cannabidiol. It lacks affinity for cannabinoid receptors, but acts as an agonist at both TRPV1 and PPARγ.[1][2][3][4]
 | |
| Identifiers | |
|---|---|
| |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C21H30O4 |
| Molar mass | 346.467 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
See also
References
- Taglialatela-Scafati O, Pagani A, Scala F, De Petrocellis L, Di Marzo V, Grassi G, Appendino G (2010). "Cannabimovone, a Cannabinoid with a Rearranged Terpenoid Skeleton from Hemp". European Journal of Organic Chemistry. 2010 (11): 2067–2072. doi:10.1002/ejoc.200901464.
- Carreras J, Kirillova MS, Echavarren AM (June 2016). "Synthesis of (-)-Cannabimovone and Structural Reassignment of Anhydrocannabimovone through Gold(I)-Catalyzed Cycloisomerization". Angewandte Chemie (International Ed. in English ed.). 55 (25): 7121–5. doi:10.1002/anie.201601834. PMC 5053274. PMID 27119910.
- Morales P, Reggio PH, Jagerovic N (2017). "An Overview on Medicinal Chemistry of Synthetic and Natural Derivatives of Cannabidiol". Frontiers in Pharmacology. 8: 422. doi:10.3389/fphar.2017.00422. PMC 5487438. PMID 28701957.
- Iannotti FA, De Maio F, Panza E, Appendino G, Taglialatela-Scafati O, De Petrocellis L, Amodeo P, Vitale RM (March 2020). "Identification and Characterization of Cannabimovone, a Cannabinoid from Cannabis sativa, as a Novel PPARγ Agonist via a Combined Computational and Functional Study". Molecules (Basel, Switzerland). 25 (5): 1119. doi:10.3390/molecules25051119. PMC 7179127. PMID 32138197.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.