HU-308
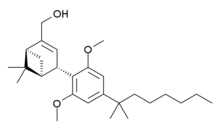
HU-308 (also known as onternabez, HU308, PPP-003 and ARDS-003) is a cannabidiol (CBD)-derivative drug that acts as a potent cannabinoid agonist. It is highly selective for the cannabinoid-2 receptor (CB2 receptor) subtype, with a selectivity more than 5,000 times greater for the CB2 receptor than the CB1 receptor.[1][2][3] The synthesis and characterization of HU-308 took place in the laboratory of Raphael Mechoulam at the Hebrew University of Jerusalem (the HU in HU-308) in the late 1990s. The pinene dimethoxy-DMH-CBD derivative HU-308 was identified as a potent peripheral CB2-selective agonist in in vitro and animal studies in 1990[1] and 1999.[2]
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Liver |
| Excretion | Kidneys |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C27H42O3 |
| Molar mass | 414.630 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Legal status
Tetra Bio-Pharma owns the intellectual property rights to HU-308.[4][5]
HU-308 is non-psychoactive and not scheduled at the federal level in the United States.[6] It is a Schedule I controlled substance in the state of Florida making it illegal to buy, sell, or possess there.[7]
References
- Mechoulam R, Lander N, Breuer A, Zahalka J (1990-04-11). "Synthesis of the individual, pharmacologically distinct enantiomers of a tetrahydrocannabinol derivative". Tetrahedron Asymmetry. 1 (5): 315–318. doi:10.1016/S0957-4166(00)86322-3.
- Hanus L, Breuer A, Tchilibon S, Shiloah S, Goldenberg D, Horowitz M, et al. (December 1999). "HU-308: a specific agonist for CB(2), a peripheral cannabinoid receptor". Proceedings of the National Academy of Sciences of the United States of America. 96 (25): 14228–14233. Bibcode:1999PNAS...9614228H. doi:10.1073/pnas.96.25.14228. PMC 24419. PMID 10588688.
- "Properties of HU-308 ~ Formula C27H42O3". Pitt Quantum Repository. University of Pittsburgh Department of Chemistry.
- US 9549906, Lynch M, Kelly M, "Composition & Methods for Treatment of Ocular Inflammation &/or Pain", issued 24 January 2017, assigned to Panag Pharma, Inc.
- "Tetra Bio-Pharma Closes the Acquisition of Panag Pharma". May 2019.
- "21 CFR — Schedules of controlled substances §1308.11 Schedule I." Archived from the original on 2009-08-27. Retrieved 2014-12-17.
- "Chapter 893 - Drug abuse prevention and control". Florida Statutes.