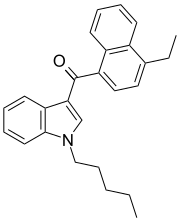
JWH-210
JWH-210 is an analgesic chemical from the naphthoylindole family, which acts as a potent cannabinoid agonist at both the CB1 and CB2 receptors, with Ki values of 0.46 nM at CB1 and 0.69 nM at CB2. It is one of the most potent 4-substituted naphthoyl derivatives in the naphthoylindole series, having a higher binding affinity (i.e. lower Ki) at CB1 than both its 4-methyl and 4-n-propyl homologues JWH-122 (CB1 Ki 0.69 nM) and JWH-182 (CB1 Ki 0.65 nM) respectively, and than the 4-methoxy compound JWH-081 (CB1 Ki 1.2 nM).[3] It was discovered by and named after John W. Huffman.
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.233.380 |
| Chemical and physical data | |
| Formula | C26H27NO |
| Molar mass | 369.508 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
JWH-210 may be neurotoxic to animals when administered in high doses.[4]
Legal status
In the United States, all CB1 receptor agonists of the 3-(1-naphthoyl)indole class such as JWH-210 are Schedule I Controlled Substances.[5]
JWH-210 and JWH-122 were banned in Sweden on 1 October 2010 as hazardous goods harmful to health, after being identified as ingredients in "herbal" synthetic cannabis products.[6][7] The substances JWH-210, JWH-122 and JWH-203 were classified as illegal drugs by the Swedish government as of 1 September 2011.[8]
As of October 2015 JWH-210 is a controlled substance in China.[9]
References
- Anvisa (2023-07-24). "RDC Nº 804 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 804 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-07-25). Archived from the original on 2023-08-27. Retrieved 2023-08-27.
- "Ustawa z dnia 15 kwietnia 2011 r. o zmianie ustawy o przeciwdziałaniu narkomanii ( Dz.U. 2011 nr 105 poz. 614 )". Internetowy System Aktów Prawnych. Retrieved 12 June 2011.
- Huffman JW, Zengin G, Wu MJ, Lu J, Hynd G, Bushell K, et al. (January 2005). "Structure-activity relationships for 1-alkyl-3-(1-naphthoyl)indoles at the cannabinoid CB(1) and CB(2) receptors: steric and electronic effects of naphthoyl substituents. New highly selective CB(2) receptor agonists". Bioorganic & Medicinal Chemistry. 13 (1): 89–112. doi:10.1016/j.bmc.2004.09.050. PMID 15582455.
- Cha HJ, Seong YH, Song MJ, Jeong HS, Shin J, Yun J, et al. (November 2015). "Neurotoxicity of Synthetic Cannabinoids JWH-081 and JWH-210". Biomolecules & Therapeutics. 23 (6): 597–603. doi:10.4062/biomolther.2015.057. PMC 4624077. PMID 26535086.
- : Schedules of controlled substances
- Swedish Code of Statutes Regulation (2010:1086).
- "Swedish Code of Statutes Regulation (2010:1086). (pdf)" (PDF). Archived from the original (PDF) on 2011-07-28. Retrieved 2011-01-10.
- LVFS 2011:8
- "关于印发《非药用类麻醉药品和精神药品列管办法》的通知" (in Chinese). China Food and Drug Administration. 27 September 2015. Archived from the original on 1 October 2015. Retrieved 1 October 2015.