2C-T-28
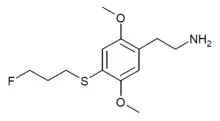
2C-T-28 is a lesser-known psychedelic drug related to compounds such as 2C-T-7 and 2C-T-21. It was named by Alexander Shulgin but was never made or tested by him, and was instead first synthesised by Daniel Trachsel some years later. It has a binding affinity of 75 nM at 5-HT2A and 28 nM at 5-HT2C. It is reportedly a potent psychedelic drug with an active dose in the 8–20 mg range, and a duration of action of 8–10 hours, with prominent visual effects. 2C-T-28 is the 3-fluoropropyl instead of 2-fluoroethyl chain-lengthened homologue of 2C-T-21 and has very similar properties, although unlike 2C-T-21 it will not form toxic fluoroacetate as a metabolite.[1][2]
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2-[4-(3-fluoropropylsulfanyl)-2,5-dimethoxyphenyl]ethanamine | |
| Identifiers | |
3D model (JSmol) |
|
PubChem CID |
|
| |
| |
| Properties | |
| C13H20FNO2S | |
| Molar mass | 273.37 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
References
- Shulgin A, Manning T, Daley PF (2011). The Shulgin Index. Volume 1. Psychedelic Phenethylamines and Related Compounds. Transform Press. p. 346. ISBN 978-0-9630096-3-0.
- Trachsel D, Lehmann D, Enzensperger C (2013). Phenethylamine: Von der Struktur zur Funktion. Nachtschatten Verlag AG. pp. 789–794. ISBN 978-3-03788-700-4.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.