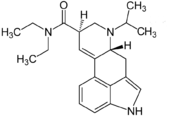
6-Isopropyl-6-nor-lysergic acid diethylamide
6-Isopropyl-6-nor-lysergic acid diethylamide (IP-LAD) is an analog of lysergic acid diethylamide (LSD) developed by the team of David E. Nichols. In studies on mice, it was found to be approximately 40% the potency of LSD, compared to the 60% increase in potency seen with ETH-LAD, 2-fold potency increase of AL-LAD, and roughly equivalent potency of PRO-LAD.[1]
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C22H29N3O |
| Molar mass | 351.494 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
References
- Hoffman AJ, Nichols DE (September 1985). "Synthesis and LSD-like discriminative stimulus properties in a series of N(6)-alkyl norlysergic acid N,N-diethylamide derivatives". Journal of Medicinal Chemistry. 28 (9): 1252–1255. doi:10.1021/jm00147a022. PMID 4032428.
| 5-HT1 |
| ||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-HT2 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT3–7 |
| ||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||
| Lysergic acid derivatives |
|
|---|---|
| Psychedelic lysergamides |
|
| Clavines | |
| Other ergolines | |
| Natural sources |
Morning glory: Argyreia nervosa (Hawaiian Baby Woodrose), Ipomoea spp.(Morning Glory, Tlitliltzin, Badoh Negro), Rivea corymbosa (Coaxihuitl, Ololiúqui) |
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.