Methylisopropyllysergamide
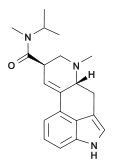
Methylisopropyllysergamide (lysergic acid methylisopropyl amide, MIPLA) is an analogue of LSD that was originally discovered by Albert Hofmann at Sandoz during the original structure-activity research into LSD. It has subsequently been investigated in more detail by the team led by David E. Nichols at Purdue University. Methylisopropyllysergamide is a structural isomer of LSD, with the alkyl groups on the amide nitrogen having been subjected to a methylene shuffle. MIPLA and its ethylisopropyl homologue are the only simple N,N-dialkyl lysergamides that approach the potency of LSD itself, being around 1/3-1/2 the potency of LSD,[2] while all other dialkyl analogues tested (dimethyl, dipropyl, methylethyl etc.) are only around 1/10 as potent as LSD,[3] although some N-monoalkyl lysergamides such as the sec-butyl and t-butyl derivatives were also found to show an activity profile and potency comparable to LSD,[4] and the mono-isopropyl derivative is only slightly weaker than MIPLA. Apart from its lower potency, the hallucinogenic effects of methylisopropyllysergamide are similar to those of LSD itself, and the main use for this drug has been in studies of the binding site at the 5-HT2A receptor through which LSD exerts most of its pharmacological effects.[5]
 | |
| Clinical data | |
|---|---|
| Other names | (6aR,9R)- N-methyl- N-isopropyl- 7-methyl- 4,6,6a,7,8,9- hexahydroindolo- [4,3-fg] quinoline- 9- carboxamide; |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| Chemical and physical data | |
| Formula | C20H25N3O |
| Molar mass | 323.440 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
References
- "Arrêté du 20 mai 2021 modifiant l'arrêté du 22 février 1990 fixant la liste des substances classées comme stupéfiants". www.legifrance.gouv.fr (in French). 20 May 2021.
- Huang X, Marona-Lewicka D, Pfaff RC, Nichols DE (March 1994). "Drug discrimination and receptor binding studies of N-isopropyl lysergamide derivatives". Pharmacology Biochemistry and Behavior. 47 (3): 667–73. doi:10.1016/0091-3057(94)90172-4. PMID 8208787. S2CID 16490010.
- Hofmann A (June 1959). "Psychotomimetic drugs; chemical and pharmacological aspects" (PDF). Acta Physiologica et Pharmacologica Neerlandica. 8: 240–58. PMID 13852489.
- US patent 2997470, Pioch RP, "Lysergic Acid Amides", published 1956-03-05, issued 1961-08-22
- Nichols DE (2001). "LSD and its lysergamide cousins" (PDF). The Heffter Review of Psychedelic Research. Santa Fe, New Mexico: Heffter Research Institute. 2: 80–7.