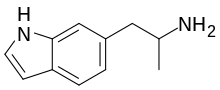
6-(2-Aminopropyl)indole
6-(2-Aminopropyl)indole (6-API, 6-IT) is an indole derivative which was first identified being sold on the designer drug market by a laboratory in the Czechia in July 2016.[2]
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C11H14N2 |
| Molar mass | 174.247 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Alexander Shulgin says in his book TiHKAL "From the normal 3-position to the 2, the 4, the 5, the 6 or the 7-positions. All five alpha-methyltryptamine isomers are known, but only one is known to be active in man as a CNS active material. This is the 5-isomer, 5-(2-aminopropyl)indole or 5-IT".
Studies in dogs have also shown the drug to increase hemoglobin levels in the bloodstream.[3]
Legality
- 6-API is a positional isomer of αMT, and as such may be covered by the analogue act in the USA (depending on the nature of its psychoactive effect).
- 6-API / 6-IT is illegal in the UK, as it was banned as a temporary class drug in June 2013, along with 9 other related compounds.[4] On March 5, 2014, the UK Home Office announced that 6-API would be made a class B drug on 10 June 2014 alongside every other benzofuran entactogen and many structurally related drugs.[5]
- 6-API is covered by the Australian analogue act as an analogue of MDA "by the replacement of up to 2 carbocyclic or heterocyclic ring structures with different carbocyclic or heterocyclic ring structures"[6]
- 6-API is uncontrolled in Germany, as indole rings are not included as rings under the 2-phenethylamine derived section of the NPsG.[1]
References
- "New Psychoactive Substances Act (Neue-psychoaktive-Stoffe-Gesetz) (NpSG) Non-official translation" (PDF). Retrieved 9 November 2021.
- Europol 2016 Annual Report on the implementation of Council Decision 2005/387/JHA
- Maxwell GM (September 1964). "The effects of an indole derivative 692'-amino-propyl indole) on the general and coronary haemodynamics of the intact dog". Experientia. 20 (9): 526–7. doi:10.1007/BF02154095. PMID 5856353. S2CID 7574163.
- "Temporary class drug order report on 5-6APB and NBOMe compounds". UK Home Office. 4 Jun 2013. Retrieved 2013-07-11.
- UK Home Office (2014-03-05). "The Misuse of Drugs Act 1971 (Ketamine etc.) (Amendment) Order 2014". UK Government. Retrieved 2014-03-11.
- "Criminal Code Act 1995" (PDF). Australian Government. 2009-08-05. Retrieved 2012-02-08.
PAGE 503
| Phenylalkyl- amines (other than cathinones) |
|
|---|---|
| Cyclized phenyl- alkylamines | |
| Cathinones | |
| Tryptamines | |
| Chemical classes | |
| D1-like |
| ||||||
|---|---|---|---|---|---|---|---|
| D2-like |
| ||||||
| |||||||
| 5-HT1 |
| ||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-HT2 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT3–7 |
| ||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||
| Phenethylamines |
|
|---|---|
| Amphetamines |
|
| Phentermines |
|
| Cathinones |
|
| Phenylisobutylamines | |
| Phenylalkylpyrrolidines | |
| Catecholamines (and close relatives) |
|
| Miscellaneous |
|
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.