Beryllium borohydride
Beryllium borohydride is an inorganic compound with the chemical formula Be(BH4)2.[2]
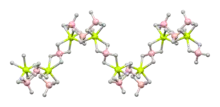 | |
 | |
| Names | |
|---|---|
| IUPAC name
Beryllium borohydride | |
| Other names
Beryllium tetrahydroborate(1−), Beryllium tetrahydroborate(III) | |
| Identifiers | |
3D model (JSmol) |
|
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| Be(BH4)2 | |
| Molar mass | 38.70 g/mol |
| Appearance | white crystals |
| Density | 0.604 g/cm3 |
| Melting point | 91.3 °C (196.3 °F; 364.4 K) |
| Boiling point | 123 °C (253 °F; 396 K) decomposes |
| reacts | |
| Solubility | soluble in benzene, diethyl ether |
| Structure | |
| tetragonal | |
| I41cd, No. 110 | |
| Thermochemistry | |
Std enthalpy of formation (ΔfH⦵298) |
-108 kJ/mol |
| Hazards | |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
TWA 0.002 mg/m3 C 0.005 mg/m3 (30 minutes), with a maximum peak of 0.025 mg/m3 (as Be)[1] |
REL (Recommended) |
Ca C 0.0005 mg/m3 (as Be)[1] |
IDLH (Immediate danger) |
Ca [4 mg/m3 (as Be)][1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Preparation
Beryllium borohydride is formed by the reaction of beryllium hydride with diborane in an ether solution.
It can also be formed by the reaction of beryllium chloride and lithium borohydride in a sealed tube at 120 °C:[2]
- BeCl2 + 2LiBH4 → BeB2H8 + 2LiCl
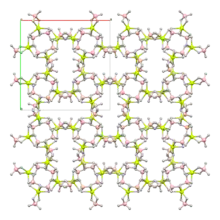
Structure
The crystal structure is made up of a helical polymer of BH4Be and BH4 structure units.[2][3] The borohydride ions, BH−4, adopt a tetrahedral geometry.[3] Beryllium is 6-coordinate and adopts a distorted trigonal prismatic geometry.[2]
Application
The purest beryllium hydride is obtained by the reaction of triphenylphosphine, PPh3, with beryllium borohydride, Be(BH4)2 at 180 °C:[2]
- Be(BH4)2 + 2 PPh3 → 2 Ph3PBH3 + BeH2
References
- NIOSH Pocket Guide to Chemical Hazards. "#0054". National Institute for Occupational Safety and Health (NIOSH).
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. pp. 115–116. ISBN 978-0-08-037941-8.
- Marynick, Dennis S.; Lipscomb, William N. (1 April 1972). "Crystal structure of beryllium borohydride". Inorg. Chem. 11 (4): 820–823. doi:10.1021/ic50110a033.
{{cite journal}}: CS1 maint: date and year (link)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.