Blastocyst
The blastocyst is a structure formed in the early embryonic development of mammals. It possesses an inner cell mass (ICM) also known as the embryoblast which subsequently forms the embryo, and an outer layer of trophoblast cells called the trophectoderm.[1][2] This layer surrounds the inner cell mass and a fluid-filled cavity known as the blastocoel.[3] In the late blastocyst the trophectoderm is known as the trophoblast.[2] The trophoblast gives rise to the chorion and amnion, the two fetal membranes that surround the embryo. The placenta derives from the embryonic chorion (the portion of the chorion that develops villi) and the underlying uterine tissue of the mother.[4][5]
| Blastocyst | |
|---|---|
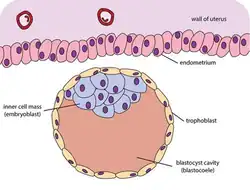 Blastocyst just before implantation | |
 A human blastocyst, with inner cell mass at upper right | |
| Details | |
| Carnegie stage | 3 |
| Days | 5–9 |
| Gives rise to | Gastrula |
| Identifiers | |
| Latin | Blastocystis |
| MeSH | D001755 |
| TE | E2.0.1.2.0.0.12 |
| FMA | 83041 |
| Anatomical terminology | |
The name "blastocyst" arises from the Greek βλαστός blastós ("a sprout") and κύστις kýstis ("bladder, capsule").
In non-mammalian animals this is a structure consisting of an undifferentiated ball of cells and is called a blastula.
In humans, blastocyst formation begins about five days after fertilization when a fluid-filled cavity opens up in the morula, the early embryonic stage of a ball of 16 cells. The blastocyst has a diameter of about 0.1–0.2 mm and comprises 200–300 cells (32 mitotic divisions ) following rapid cleavage (cell division). About seven days after fertilization,[6] the blastocyst undergoes implantation, embedding into the endometrium of the uterine wall where it will undergo further developmental processes, including gastrulation. Embedding of the blastocyst into the endometrium requires that it hatches from the zona pellucida, the egg coat that prevents adherence to the fallopian tube as the pre-embryo makes its way to the uterus.
The use of blastocysts in in vitro fertilization (IVF) involves culturing a fertilized egg for five days before transferring it into the uterus. It can be a more viable method of fertility treatment than traditional IVF. The inner cell mass of blastocysts is the source of embryonic stem cells, which are broadly applicable in stem cell therapies including cell repair, replacement and regeneration. Assisted zona hatching may also be used in IVF, and other fertility treatments.
Development cycle
The blastocyst stage occurs between 5 and 9 days after conception. During embryonic development, after fertilization (approximately 5–6 days in the human), the cells of the morula begin to undergo cell differentiation, and the morula changes into the blastocyst. In the uterus the zona pellucida surrounding the blastocyst breaks down, allowing it to implant into the uterine wall. Implantation marks the end of the germinal stage of embryogenesis, and the beginning of gestation.
Blastocyst formation

The zygote develops by mitosis, and when it has developed into a compacted ball of 8-16 cells becomes known as the morula. Until this stage in development, all cells (blastomeres) are autonomous and not specified to any particular subsequent functional development. The morula then develops by cavitation to become the blastocyst, or in many other animals the blastula. Cellular differentiation then develops the morula's cells into two types: trophoblast cells that surround the blastocoel and an inner mass of cells (the embryoblast). The inner cell mass consists of embryonic stem cells.[7] The conceptus is then known as the blastocyst.[8]
Before cell differentiation takes place there are two transcription factors, Oct-4 and nanog that are uniformly expressed on all of the cells, but both of these transcription factors are turned off in the trophoblast once it has formed.[9] The side of the blastocyst where the inner cell mass forms is called the embryonic pole, and the opposite side is the abembryonic pole. The outer layer of trophoblast cells, resulting from compaction, pumps sodium ions into the blastocyst, which causes water to enter through osmosis and form the internal fluid-filled blastocyst cavity (blastocoel). The blastocoel, trophoblast cells, and inner cell mass are hallmarks of the blastocyst.[10]
Implantation
Implantation is critical to the survival and development of the early human embryo. It establishes a connection between the mother and the early embryo which will continue through the remainder of the pregnancy. Implantation is made possible through structural changes in both the blastocyst and endometrial wall.[11] The zona pellucida surrounding the blastocyst breaches, referred to as hatching. This removes the constraint on the physical size of the embryonic mass and exposes the outer cells of the blastocyst to the interior of the uterus. Furthermore, hormonal changes in the mother, specifically a peak in luteinizing hormone (LH), prepare the endometrium to receive and envelop the blastocyst. The immune system is also modulated to allow for the invasion of the foreign embryonic cells. Once bound to the extracellular matrix of the endometrium, trophoblast cells secrete enzymes and other factors to embed the blastocyst into the uterine wall. The enzymes released degrade the endometrial lining, while autocrine growth factors such as human chorionic gonadotropin (hCG) and insulin-like growth factor (IGF) allow the blastocyst to further invade the endometrium.[12]
Implantation in the uterine wall allows for the next step in embryogenesis, gastrulation, which includes the formation of the placenta from trophoblastic cells and differentiation of the inner cell mass into the amniotic sac and epiblast.
Structure
There are two types of blastomere cells:[13]
- The inner cell mass, also known as the embryoblast, gives rise to the primitive endoderm and the embryo proper (epiblast).
- The primitive endoderm develops into the amniotic sac which forms the fluid-filled cavity that the embryo resides in during pregnancy.[14]
- The epiblast gives rise to the three germ layers of the developing embryo during gastrulation (endoderm, mesoderm, and ectoderm).
- The trophoblast is a layer of cells forming the outer ring of the blastocyst that combines with the maternal endometrium to form the placenta. Trophoblast cells also secrete factors to make the blastocoel.[15]
- After implantation, cytotrophoblast is the inner layer of the trophoblast, composed of stem cells which give rise to cells comprising the chorionic villi, placenta, and syncytiotrophoblast.
- After implantation, syncytiotrophoblast is the outermost layer of the trophoblast. These cells secrete proteolytic enzymes to break down the endometrial extracellular matrix to allow for implantation of the blastocyst in the uterine wall.[16]
The blastocoel fluid cavity contains amino acids, growth factors, and other molecules necessary for cellular differentiation.[17]
Cell specification
Multiple processes control cell lineage specification in the blastocyst to produce the trophoblast, epiblast, and primitive endoderm. These processes include gene expression, cell signaling, cell-cell contact and positional relationships, and epigenetics.
Once the inner cell mass has been established within the blastocyst, it prepares for further specification into the epiblast and primitive endoderm. This process of specification known as cell fate determination is carried out in part by fibroblast growth factor (FGF) signaling which generates a MAP kinase pathway to alter cellular genomes.[18] Further segregation of blastomeres into the trophectoderm and inner cell mass are regulated by the homeodomain protein, Cdx2. This transcription factor represses the expression of Oct4 and Nanog transcription factors in the trophoblast.[19] These genomic alterations allow for the progressive specification of both epiblast and primitive endoderm lineages at the end of the blastocyst phase of development preceding gastrulation. Much of the research conducted on these early embryonic stages is on mouse embryos and specific factors may differ between mammals.
During implantation, the trophoblast gives rise to extraembryonic membranes and cell types that will eventually form most of the fetal placenta, the specialized organ through which the embryo obtains maternal nourishment necessary for subsequent exponential growth.[20] The specification of the trophoblast is controlled by the combination of morphological cues arising from cell polarity with differential activity of signaling pathways such as Hippo and Notch, and the restriction to outer cells of lineage specifiers such as CDX2.[21]
In the mouse, primordial germ cells are specified from epiblast cells, a process that is accompanied by extensive genome-wide epigenetic reprogramming.[22] Reprogramming involves global DNA demethylation and chromatin reorganization resulting in cellular totipotency.[22] The process of genome-wide demethylation involves the DNA base excision repair pathway.[23]
Trophoblasts express integrin on their cell surfaces which allow for adhesion to the extracellular matrix of the uterine wall. This interaction allows for implantation and triggers further specification into the three different cell types, preparing the blastocyst for gastrulation.[24]
Clinical implications
Pregnancy tests
The level of human chorionic gonadotropin (hCG) secreted by the blastocyst during implantation is the factor measured in a pregnancy test. hCG can be measured in both blood and urine to determine whether a woman is pregnant. More hCG is secreted in a multiple pregnancy. Blood tests of hCG can also be used to check for abnormal pregnancies.
In vitro fertilization
In vitro fertilization (IVF) is an alternative to traditional in vivo fertilization for fertilizing an egg with sperm and implanting that embryo into a female's womb. For many years the embryo was inserted into the uterus two to three days after fertilization. However at this stage of development it is very difficult to predict which embryos will develop best, and several embryos were typically implanted. Several implanted embryos increased the likelihood of a developing fetus but also led to the development of multiple fetuses. This was a major problem and drawback for using embryos in IVF.
The use of blastocysts for human IVF has proved successful. A blastocyst is implanted five to six days after the eggs have been fertilized.[25] After five or six days it is much easier to determine which embryos will result in healthy live births. Knowing which embryos will succeed allows just one blastocyst to be implanted, cutting down dramatically on the health risk and expense of multiple births. Now that the nutrient requirements for embryonic and blastocyst development have been determined, it is much easier to give embryos the correct nutrients to sustain them into the blastocyst phase.
Embryo transfer following in vitro fertilization is a procedure in which a catheter is inserted into the vagina, guided through the cervix via ultrasound, and into the uterine cavity where the blastocysts are inserted into the womb.
Blastocysts also offer an advantage because they can be used to genetically test the cells to check for genetic problems. There are enough cells in a blastocyst that a few trophectoderm cells can be removed without disturbing the developing blastocyst. These cells can be tested for chromosome aneuploidy using preimplantation genetic screening (PGS), or specific conditions such as cystic fibrosis, often known as preimplantation genetic diagnosis (PGD).[26]
Embryo transfer process
In an embryo transfer procedure following an initial ultrasound, a speculum is used to open the walls of the vagina, and using a catheter an embryo is passed through the tube for placement into the womb.
See also
References
![]() This article incorporates text in the public domain from the 20th edition of Gray's Anatomy (1918)
This article incorporates text in the public domain from the 20th edition of Gray's Anatomy (1918)
- "27.2C: Blastocyst Formation". Medicine LibreTexts. 24 July 2018. Retrieved 11 October 2022.
- Standring, Susan (2016). Gray's anatomy : the anatomical basis of clinical practice (Forty-first ed.). [Philadelphia]: Elsevier Limited. p. 167. ISBN 9780702052309.
- Gilbert, Scott F. (2000). "Early Mammalian Development". Developmental Biology. 6th edition. Retrieved 13 May 2022.
- "trophoblast | embryology". Encyclopedia Britannica. Retrieved 2021-11-01.
- Solomon, Eldra (2018). Biology 11th Edition. Cengage Learning. ISBN 978-1337392938.
- VanPutte C (2020). Seeley's Anatomy & Physiology. New York: McGraw-Hill. p. 1092. ISBN 978-1-260-56596-6. OCLC 1099344977.
- Molnar, Charles; Gair, Jane (14 May 2015). "24.6. Fertilization and Early Embryonic Development". Retrieved 19 October 2022.
- Nissen SB, Perera M, Gonzalez JM, Morgani SM, Jensen MH, Sneppen K, et al. (July 2017). "Four simple rules that are sufficient to generate the mammalian blastocyst". PLOS Biology. 15 (7): e2000737. doi:10.1371/journal.pbio.2000737. PMC 5507476. PMID 28700688.
- Schoenwolf, Gary C. (2015). Larsen's human embryology (Fifth ed.). Philadelphia, PA. pp. 35–37. ISBN 9781455706846.
{{cite book}}: CS1 maint: location missing publisher (link) - Gilbert SF (2000). "Early Mammalian Development". Developmental Biology (6th ed.). Sunderland (MA): Sinauer Associates.
- Zhang S, Lin H, Kong S, Wang S, Wang H, Wang H, Armant DR (October 2013). "Physiological and molecular determinants of embryo implantation". Molecular Aspects of Medicine. 34 (5): 939–80. doi:10.1016/j.mam.2012.12.011. PMC 4278353. PMID 23290997.
- Srisuparp S, Strakova Z, Fazleabas AT (2001). "The role of chorionic gonadotropin (CG) in blastocyst implantation". Archives of Medical Research. 32 (6): 627–34. doi:10.1016/S0188-4409(01)00330-7. PMID 11750740.
- Gilbert SF (15 July 2013). Developmental Biology. Sinauer Associates, Incorporated. ISBN 978-1-60535-173-5.
- Schoenwolf GC, Larsen WJ (2009). Larsen's Human Embryology (4th ed.). Philadelphia: Churchill Livingstone/Elsevier.
- James JL, Stone PR, Chamley LW (July 2005). "Cytotrophoblast differentiation in the first trimester of pregnancy: evidence for separate progenitors of extravillous trophoblasts and syncytiotrophoblast". Reproduction. 130 (1): 95–103. doi:10.1530/rep.1.00723. PMID 15985635.
- Vićovac L, Aplin JD (1996). "Epithelial-mesenchymal transition during trophoblast differentiation". Acta Anatomica. 156 (3): 202–16. doi:10.1159/000147847. PMID 9124037.
- Gasperowicz M, Natale DR (April 2011). "Establishing three blastocyst lineages--then what?". Biology of Reproduction. 84 (4): 621–30. doi:10.1095/biolreprod.110.085209. PMID 21123814.
- Yamanaka Y, Lanner F, Rossant J (March 2010). "FGF signal-dependent segregation of primitive endoderm and epiblast in the mouse blastocyst". Development. 137 (5): 715–24. doi:10.1242/dev.043471. PMID 20147376. S2CID 28481311.
- Strumpf D, Mao CA, Yamanaka Y, Ralston A, Chawengsaksophak K, Beck F, Rossant J (May 2005). "Cdx2 is required for correct cell fate specification and differentiation of trophectoderm in the mouse blastocyst". Development. 132 (9): 2093–102. doi:10.1242/dev.01801. PMID 15788452.
- Menchero S, Sainz de Aja J, Manzanares M (2018). "Our First Choice: Cellular and Genetic Underpinnings of Trophectoderm Identity and Differentiation in the Mammalian Embryo". Current Topics in Developmental Biology. Elsevier. 128: 59–80. doi:10.1016/bs.ctdb.2017.10.009. hdl:20.500.12105/10490. ISBN 978-0-12-804252-6. PMID 29477171.
- Menchero S, Rollan I, Lopez-Izquierdo A, Andreu MJ, Sainz de Aja J, Kang M, et al. (April 2019). "Transitions in cell potency during early mouse development are driven by Notch". eLife. 8: e42930. doi:10.7554/eLife.42930. PMC 6486152. PMID 30958266.
- Hackett JA, Sengupta R, Zylicz JJ, Murakami K, Lee C, Down TA, Surani MA (January 2013). "Germline DNA demethylation dynamics and imprint erasure through 5-hydroxymethylcytosine". Science. 339 (6118): 448–52. Bibcode:2013Sci...339..448H. doi:10.1126/science.1229277. PMC 3847602. PMID 23223451.
- Hajkova P, Jeffries SJ, Lee C, Miller N, Jackson SP, Surani MA (July 2010). "Genome-wide reprogramming in the mouse germ line entails the base excision repair pathway". Science. 329 (5987): 78–82. Bibcode:2010Sci...329...78H. doi:10.1126/science.1187945. PMC 3863715. PMID 20595612.
- Damsky CH, Librach C, Lim KH, Fitzgerald ML, McMaster MT, Janatpour M, et al. (December 1994). "Integrin switching regulates normal trophoblast invasion". Development. 120 (12): 3657–66. doi:10.1242/dev.120.12.3657. PMID 7529679. Archived from the original on 2020-05-27. Retrieved 2013-12-10.
- Fong CY, Bongso A, Ng SC, Anandakumar C, Trounson A, Ratnam S (March 1997). "Ongoing normal pregnancy after transfer of zona-free blastocysts: implications for embryo transfer in the human". Human Reproduction. 12 (3): 557–60. doi:10.1093/humrep/12.3.557. PMID 9130759.
- Wang J, Sauer MV (December 2006). "In vitro fertilization (IVF): a review of 3 decades of clinical innovation and technological advancement". Therapeutics and Clinical Risk Management. 2 (4): 355–64. doi:10.2147/tcrm.2006.2.4.355. PMC 1936357. PMID 18360648.
External links