Epicuticular wax
Epicuticular wax is a waxy coating which covers the outer surface of the plant cuticle in land plants. It may form a whitish film or bloom on leaves, fruits and other plant organs. Chemically, it consists of hydrophobic organic compounds, mainly straight-chain aliphatic hydrocarbons with or without a variety of substituted functional groups. The main functions of the epicuticular wax are to decrease surface wetting and moisture loss. Other functions include reflection of ultraviolet light, assisting in the formation of an ultra-hydrophobic and self-cleaning surface and acting as an anti-climb surface.
Chemical composition
Common constituents of epicuticular wax are predominantly straight-chain aliphatic hydrocarbons that may be saturated or unsaturated and contain a variety of functional groups, such as -hydroxyl, carboxyl, and -ketoyl at the terminal position. This broadens the spectrum of wax composition to fatty acids, primary alcohols, and aldehydes; if the substitution occurs at the mid-chain, it will result in β-diketones and secondary alcohols.[1] Other major components of epicuticular waxes are long-chain n-alkanoic acids such as C24, C26, and C28.[2]
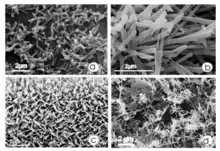
These waxes can be composed of a variety of compounds which differ between plant species. Wax tubules and wax platelets often have chemical as well as morphological differences. Tubules can be separated into two groups; the first primarily containing secondary alcohols, and the second containing β-diketones. Platelets are either dominated by triterpenoids, alkanes, aldehydes, esters, secondary alcohols, or flavonoids. However, chemical composition is not diagnostic of a tubule or platelet, as this does not determine the morphology.[3]
Paraffins occur in leaves of peas and cabbages, for example. Leaves of carnauba palm and banana feature alkyl esters. The asymmetrical secondary alcohol 10-nonacosanol appears in most gymnosperms such as Ginkgo biloba and Sitka spruce as well as many of the Ranunculaceae, Papaveraceae and Rosaceae and some mosses. Symmetrical secondary alcohols are found in Brassicaceae including Arabidopsis thaliana. Primary alcohols (most commonly octacosan-1-ol) occur in Eucalyptus, legumes, and most Poaceae grasses. Grasses may also feature β-diketones, as do Eucalyptus, box Buxus and the Ericaceae. Young beech leaves, sugarcane culms and lemon fruit exhibit aldehydes. Triterpenes are the primary component in fruit waxes of apple, plum and grape.[1][4] Cyclic constituents are often recorded in epicuticular waxes, as in Nicotiana but are generally minor constituents. They may include phytosterols such as β-sitosterol and pentacyclic triterpenoids such as ursolic acid and oleanolic acid and their respective precursors, α-amyrin and β-amyrin.[1]
Farina
Many species of the genus Primula and ferns such as Cheilanthes, Pityrogramma and Notholaena produce a mealy, whitish to pale yellow glandular secretion known as farina that is not an epicuticular wax, but consists largely of crystals of a different class of polyphenolic compounds known as flavonoids.[5] Unlike epicuticular wax, farina is secreted by specialised glandular hairs, rather than by the cuticle of the entire epidermis.[5]
Physical properties
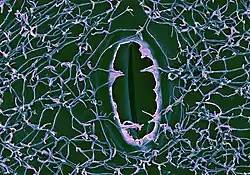
Epicuticular waxes are mostly solids at ambient temperature, with melting points above about 40 °C (100 °F). They are soluble in organic solvents such as chloroform and hexane, making them accessible for chemical analysis, but in some species esterification of acids and alcohols into estolides or the polymerization of aldehydes may give rise to insoluble compounds. Solvent extracts of cuticle waxes contain both epicuticular and cuticular waxes, often contaminated with cell membrane lipids of underlying cells. Epicuticular wax can now also be isolated by mechanical methods that distinguish the epicuticular wax outside the plant cuticle from the cuticular wax embedded in the cuticle polymer.[6] As a consequence, these two are now known to be chemically distinct,[7] although the mechanism that segregates the molecular species into the two layers is unknown. Recent scanning electron microscopy (SEM), atomic force microscopy (AFM) and neutron reflectometry studies on reconstituted wax films have found wheat epicuticular waxes;[8] made up of surface epicuticular crystals and an underlying, porous background film layer to undergo swelling when in contact with water, indicating the background film is permeable and susceptible to the transport of water.
Epicuticular wax can reflect UV light, such as the white, chalky, wax coating of Dudleya brittonii, which has the highest ultraviolet light (UV) reflectivity of any known naturally occurring biological substance.[9]
The term 'glaucous' is used to refer to any foliage, such as that of the family Crassulaceae, which appears whitish because of the waxy covering. Coatings of epicuticular flavonoids may be referred to as 'farina', the plants themselves being described as 'farinose' or 'farinaceous'.[10]: 51
Epicuticular wax crystals
Epicuticular wax forms crystalline projections from the plant surface, which enhance their water repellency,[11] create a self-cleaning property known as the lotus effect[12] and reflect UV radiation. The shapes of the crystals are dependent on the wax compounds present in them. Asymmetrical secondary alcohols and β-diketones form hollow wax nanotubes, while primary alcohols and symmetrical secondary alcohols form flat plates[13][14] Although these have been observed using the transmission electron microscope[13][15] and scanning electron microscope[16] the process of growth of the crystals had never been observed directly until Koch and coworkers[17][18] studied growing wax crystals on leaves of snowdrop (Galanthus nivalis) and other species using the atomic force microscope. These studies show that the crystals grow by extension from their tips, raising interesting questions about the mechanism of transport of the molecules.
Measurement techniques
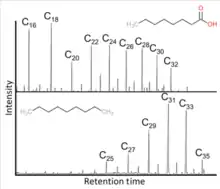
Epicuticular waxes are recovered from terrestrial, marine, and lake environments, allowing for solvent extraction of biomarkers and then qualitative and quantitative profiling through Gas Chromatography Mass Spectrometry (GC-MS) and GC Flame Ionization Detection (GC-FID). GC-MS and GC-FID are preferential for identifying and quantifying n-alkanes and n-alkanoic acids. Isotope ratio analysis (GC-IRMS) measures relative abundance of carbon, hydrogen, and other isotopes with high precision. The carbon isotopic ratio is expressed between carbon-13 and carbon-12 as δ13C relative to the international standard. The hydrogen isotopic ratio between deuterium and protium is expressed as δD relative to the international standard.[19]
Use as a biomarker
[19] Epicuticular wax has been used as a biomarker to observe human evolution patterns. These lipids of these plant waxes have been analyzed when extracted from ocean and lake cores, paleo-lake drilling projects, archeological and geological outcrops, cave deposits, and human-bearing sediments. This data provides insight into past plant ecology and environmental stresses, particularly by reconstructing landscapes at a high taxonomic resolution.
Epicuticular wax δ13C is a favorable biomarker due to its benefits: it is not biased towards feeding like tooth enamel biomarkers, and are more widespread than paleosol carbonates that are biased based on rainfall amount. This marker can also identify C3 and C4 photosynthetic pathways. Biosynthesis of these lipids result in further fractionation that results in lighter the bulk δ13C. Isotope stability studies that characterize diagenetic process can identify carbon and hydrogen alteration through chemical and microbial activity, but these studies often have mixed results. The state of plant wax preservation in soils and sediments is still unknown due to complex interactions in the depositional environments, including pH, microbial communities, alkalinity, temperature, and oxygen/moisture content.
δ13C of higher order plants has been used at Holocene and Pleistocene archeological sites. Diverse environments in modern Africa have been analyzed through the interpretation of epicuticular wax proxies, from wooded grassland vegetation (where the C31 homolog is most abundant) to arid and semi-arid regions of southern Africa (characterized by an abundance of C29). Turkana paleo-lake sediments from the East (3.45-3.4 Ma Wargolo Formation) and the West (1.9-1.4 Ma Nachukui Formation) suggest precession-controlled summer insolation is the primary driver of Pliocene and Pleistocene hydrology in the Basin. Variance of δD and δ13C at certain dates coincide with changes in variables such as orbital eccentricity and hominid tools.[19]
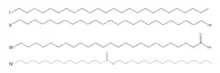
Epicuticular wax and its successor aliphatic compounds are also used as biomarkers for higher plants. Long-chain n-alkyl compounds from vascular plants leaves are major components of epicuticular waxes that are resistant to degradation and thus effective biomarkers for higher plants. These terrestrial biomarkers can also be present in marine sediments. Due to the lack of higher plant material in aqueous settings, the presence of higher plant biomarkers in these ecosystems infer that these biomarkers were transported from their original terrestrial environment. Carbon isotopic compositions, specifically, their δ13C value, reflect their metabolism and environment, as 13C is discriminated against during photosynthesis.[20]
See also
References
- Baker, E. A. (1982). "Chemistry and morphology of plant epicuticular waxes". In Cutler, D. J.; Alvin, K. L.; Price, C. E. (eds.). The Plant Cuticle. London: Academic Press. pp. 139–165. ISBN 0-12-199920-3.
- Peters, K. E.; Walters, C. C.; Moldowan, J. M. (2005). The Biomarker Guide. Vol. 1 (2nd ed.). Cambridge University Press. p. 47. ISBN 0521781582.
- Koch, Kerstin; Barthlott, Wilhelm (2006). "Plant Epicuticular Waxes: Chemistry, Form, Self-Assembly and Function". Natural Product Communications. 1 (11): 1934578X0600101. doi:10.1177/1934578X0600101123. ISSN 1934-578X – via SagePub.
- Holloway, P.J.; Jeffree, C.E. (2005). "Epicuticular waxes". Encyclopedia of Applied Plant Sciences. 3: 1190–1204.
- Walter C. Blasdale (1945). "The composition of the solid secretion produced by Primula denticulata". Journal of the American Chemical Society. 67 (3): 491–493. doi:10.1021/ja01219a036.
- Ensikat, H. J.; et al. (2000). "Direct access to plant epicuticular wax crystals by a new mechanical isolation method". International Journal of Plant Sciences. 161 (1): 143–148. doi:10.1086/314234.
- Jetter, R.; et al. (2000). "Leaf cuticular waxes are arranged in chemically and mechanically distinct layers: evidence from Prunus laurocerasus L. Plant". Cell and Environment. 23 (6): 619–628. doi:10.1046/j.1365-3040.2000.00581.x.
- Pambou, E.; et al. (2016). "Structural features of reconstituted wheat wax films". J. R. Soc. Interface. 13. 20160396. doi:10.1098/rsif.2016.0396.
- Mulroy, Thomas W. (1979). "Spectral properties of heavily glaucous and non-glaucous leaves of a succulent rosette-plant". Oecologia. 38 (3): 349–357. doi:10.1007/BF00345193. PMID 28309493. S2CID 23753011.
- Henk Beentje (2016). The Kew plant glossary (2 ed.). Richmond, Surrey: Kew Publishing. ISBN 978-1-84246-604-9.
- Holloway, P. J. (1969). "The effects of superficial wax on leaf wettability". Annals of Applied Biology. 63 (1): 145–153. doi:10.1111/j.1744-7348.1969.tb05475.x.
- Barthlott, W.; Neinhuis, C. (1997). "Purity of the sacred lotus, or escape from contamination in biological surfaces". Planta. 202: 1–8. doi:10.1007/s004250050096.
- Hallam, N. D. (1967). An electron microscope study of the leaf waxes of the genus Eucalyptus L'Heritier (PhD thesis). University of Melbourne. OCLC 225630715.
- Jeffree, C. E.; Baker, E. A.; Holloway, P. J. (1975). "Ultrastructure and recrystallisation of plant epicuticular waxes". New Phytologist. 75: 539–549. doi:10.1111/j.1469-8137.1975.tb01417.x.
- Juniper, B. E.; Bradley, D. E. (1958). "The carbon replica technique in the study of the ultrastructure of leaf surfaces". Journal of Ultrastructure Research. 2: 16–27. doi:10.1016/S0022-5320(58)90045-5.
- Jeffree, C. E. (2006). "The fine structure of the Plant Cuticle". In Riederer, M.; Müller, C. (eds.). Biology of the Plant Cuticle. Blackwell Publishing. pp. 11–125. Archived from the original on April 6, 2007.
- Koch, K.; et al. (2004). "Self assembly of epicuticular waxes on living plant surfaces imaged by atomic force microscopy (AFM)". Journal of Experimental Botany. 55: 711–718. doi:10.1093/jxb/erh077.
- Koch, K.; et al. (2005). "Structural analysis of wheat wax (Triticum aestivum, c.v. 'Naturastar' L.): from the molecular level to three dimensional crystals". Planta. 223: 258–270. doi:10.1007/s00425-005-0081-3.
- Patalano, Robert; Roberts, Patrick; Boivin, Nicole; Petraglia, Michael D.; Mercader, Julio (2021). "Plant wax biomarkers in human evolutionary studies". Evolutionary Anthropology: Issues, News, and Reviews. 30 (6): 385–398. doi:10.1002/evan.21921. hdl:10072/409183. ISSN 1060-1538 – via Wiley Online Library.
- Pancost, Richard D.; Boot, Christopher S. (2004-12-01). "The palaeoclimatic utility of terrestrial biomarkers in marine sediments". Marine Chemistry. New Approaches in Marine Organic Biogeochemistry: A Tribute to the Life and Science of John I. Hedges. 92 (1): 239–261. doi:10.1016/j.marchem.2004.06.029. ISSN 0304-4203.
Bibliography
- Eigenbrode, S. D. (1996). "Plant surface waxes and insect behaviour". In Kerstiens, G. (ed.). Plant Cuticles: an integrated functional approach. Oxford: Bios Scientific Publishers. pp. 201–221. ISBN 1-85996-130-4.