Troxerutin
Troxerutin is a flavonol, a type of flavonoid, derived from rutin.[1] It is more accurately a hydroxyethylrutoside. It can be isolated from Sophora japonica, the Japanese pagoda tree.
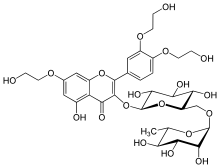 | |
| Clinical data | |
|---|---|
| Other names | Hydroxyethylrutoside (HER) Pherarutin Trihydroxyethylrutin 3',4',7-Tris[O-(2-hydroxyethyl)]rutin |
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C33H42O19 |
| Molar mass | 742.680 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
It is used as a vasoprotective.[2]
Troxerutin has been shown in mice to reverse CNS insulin resistance and reduce reactive oxygen species induced by a high-cholesterol diet.[3]
References
- Zhang ZF, Fan SH, Zheng YL, Lu J, Wu DM, Shan Q, Hu B (September 2014). "Troxerutin improves hepatic lipid homeostasis by restoring NAD(+)-depletion-mediated dysfunction of lipin 1 signaling in high-fat diet-treated mice". Biochemical Pharmacology. 91 (1): 74–86. doi:10.1016/j.bcp.2014.07.002. PMID 25026599.
- Riccioni C, Sarcinella R, Izzo A, Palermo G, Liguori M (February 2004). "[Effectiveness of Troxerutin in association with Pycnogenol in the pharmacological treatment of venous insufficiency]". Minerva Cardioangiologica. 52 (1): 43–8. PMID 14765037.
- Lu J, Wu DM, Zheng ZH, Zheng YL, Hu B, Zhang ZF (March 2011). "Troxerutin protects against high cholesterol-induced cognitive deficits in mice". Brain. 134 (Pt 3): 783–97. doi:10.1093/brain/awq376. PMID 21252113.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.