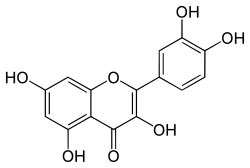
Quercetin
Quercetin is a plant flavonol from the flavonoid group of polyphenols. It is found in many fruits, vegetables, leaves, seeds, and grains; capers, red onions, and kale are common foods containing appreciable amounts of it.[2][3] It has a bitter flavor and is used as an ingredient in dietary supplements, beverages, and foods.
 | |
 | |
| Names | |
|---|---|
| Pronunciation | /ˈkwɜːrsɪtɪn/ |
| IUPAC name
3,3′,4′,5,7-Pentahydroxyflavone | |
| Systematic IUPAC name
2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-4H-1-benzopyran-4-one | |
| Other names
5,7,3′,4′-flavon-3-ol, Sophoretin, Meletin, Quercetine, Xanthaurine, Quercetol, Quercitin, Quertine, Flavin meletin | |
| Identifiers | |
3D model (JSmol) |
|
| 317313 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.003.807 |
| EC Number |
|
| 579210 | |
| KEGG | |
PubChem CID |
|
| UNII |
|
| UN number | 2811 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C15H10O7 | |
| Molar mass | 302.236 g/mol |
| Appearance | yellow crystalline powder[1] |
| Density | 1.799 g/cm3 |
| Melting point | 316 °C (601 °F; 589 K) |
| Practically insoluble in water; soluble in aqueous alkaline solutions[1] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Occurrence
Quercetin is a flavonoid widely distributed in nature.[2] The name has been used since 1857, and is derived from quercetum (oak forest), after the oak genus Quercus.[4][5] It is a naturally occurring polar auxin transport inhibitor.[6]
Quercetin is one of the most abundant dietary flavonoids,[2][3] with an average daily consumption of 25–50 milligrams.[7]
| Foods | Quercetin, mg / 100 g |
|---|---|
| capers, raw | 234[3] |
| capers, canned | 173[3] |
| lovage leaves, raw | 170[3] |
| dock like sorrel | 86[3] |
| radish leaves | 70[3] |
| carob fiber | 58[3] |
| dill weed, fresh | 55[3] |
| coriander | 53[3] |
| yellow wax pepper, raw | 51[3] |
| fennel leaves | 49[3] |
| onion, red | 32[3] |
| radicchio | 32[3] |
| watercress | 30[3] |
| kale | 23[3] |
| chokeberry | 19[3] |
| bog blueberry | 18[3] |
| buckwheat seeds | 15[3] |
| cranberry | 15[3] |
| lingonberry | 13[3] |
| plums, black | 12[3] |
In red onions, higher concentrations of quercetin occur in the outermost rings and in the part closest to the root, the latter being the part of the plant with the highest concentration.[8] One study found that organically grown tomatoes had 79% more quercetin than non-organically grown fruit.[9] Quercetin is present in various kinds of honey from different plant sources.[10]
Biosynthesis
In plants, phenylalanine is converted to 4-coumaroyl-CoA in a series of steps known as the general phenylpropanoid pathway using phenylalanine ammonia-lyase, cinnamate-4-hydroxylase, and 4-coumaroyl-CoA-ligase.[11] One molecule of 4-coumaroyl-CoA is added to three molecules of malonyl-CoA to form tetrahydroxychalcone using 7,2′-dihydroxy-4′-methoxyisoflavanol synthase. Tetrahydroxychalcone is then converted into naringenin using chalcone isomerase.
Naringenin is converted into eriodictyol using flavanoid 3′-hydroxylase. Eriodictyol is then converted into dihydroquercetin with flavanone 3-hydroxylase, which is then converted into quercetin using flavonol synthase.[11]
Glycosides
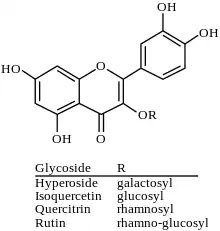
Quercetin is the aglycone form of a number of other flavonoid glycosides, such as rutin (also known as quercetin-3-O-rutinoside) and quercitrin, found in citrus fruit, buckwheat and onions.[2] Quercetin forms the glycosides quercitrin and rutin together with rhamnose and rutinose, respectively. Likewise guaijaverin is the 3-O-arabinoside, hyperoside is the 3-O-galactoside, isoquercitin is the 3-O-glucoside and spiraeoside is the 4′-O-glucoside. CTN-986 is a quercetin derivative found in cottonseeds and cottonseed oil. Miquelianin is the quercetin 3-O-β-D-glucuronopyranoside.[12]
A number of taxifolin (also known as dihydroquercetin) glycosides also exists.
Isoquercetin is the 3-O-glucoside of quercetin.
Rutin degradation pathway
The enzyme quercitrinase can be found in Aspergillus flavus.[13] This enzyme hydrolyzes the glycoside quercitrin to release quercetin and L-rhamnose. It is an enzyme in the rutin catabolic pathway.[14]
Pharmacology
Pharmacokinetics
The bioavailability of quercetin in humans after oral intake is very low, with one study concluding it must be less than 1%.[15] Intravenous injection of quercetin shows a rapid decay in concentration described by a two-compartment model (initial half-life of 8.8 minutes, terminal half-life of 2.4 hours).[15] Because it undergoes rapid and extensive metabolism, the biological effects presumed from in vitro studies are unlikely to apply in vivo.[2][16][17][18] Quercetin supplements in the aglycone form are less bioavailable than the quercetin glycoside often found in foods, especially red onions.[2][19] Ingestion with high-fat foods may increase bioavailability compared to ingestion with low-fat foods,[19] and carbohydrate-rich foods may increase absorption of quercetin by stimulating gastrointestinal motility and colonic fermentation.[2]
Metabolism
Quercetin is rapidly metabolized (via glucuronidation) after the ingestion of quercetin foods or supplements.[20] Five metabolites (quercetin glucuronides) have been found in human plasma after quercetin ingestion.[21][20] Taken together, the quercetin glucuronides have a half life around 11-12 hours.[20]
In rats, quercetin did not undergo any significant phase I metabolism.[22] In contrast, quercetin did undergo extensive phase II (conjugation) to produce metabolites that are more polar than the parent substance and hence are more rapidly excreted from the body. In vitro, the meta-hydroxyl group of catechol is methylated by catechol-O-methyltransferase. Four of the five hydroxyl groups of quercetin are glucuronidated by UDP-glucuronosyltransferase. The exception is the 5-hydroxyl group of the flavonoid ring which generally does not undergo glucuronidation. The major metabolites of orally absorbed quercetin are quercetin-3-glucuronide, 3'-methylquercetin-3-glucuronide, and quercetin-3'-sulfate.[22] A methyl metabolite of quercetin has been shown in vitro to be more effective than quercetin at inhibiting lipopolysaccharide-activated macrophages.[18]
Compared to other flavonoids quercetin is one of the most effective inducers of the phase II detoxification enzymes.[23]
In-vitro studies show that quercetin is a strong inhibitor of the cytochrome P450 enzymes CYP3A4 and CYP2C19 and a moderate inhibitor of CYP2D6.[24][25] Drugs that are metabolized by these pathways may have increased effect. An in-vivo study found that quercetin supplementation slows the metabolism of caffeine to a statistically significant extent in a particular genetic sub-population, but in absolute terms the effect was almost negligible.[26]
Pharmacological research
Quercetin has been reported to inhibit the oxidation of other molecules and hence is classified as an antioxidant in vitro.[16] It contains a polyphenolic chemical substructure that stops oxidation in vitro by acting as a scavenger of free radicals. Quercetin has been shown to inhibit the PI3K/AKT pathway leading to downregulation of the anti-apoptotic protein Bcl-w.[27][28] Quercetin activates or inhibits the activities of a number of proteins in vitro. For example, it is a nonspecific protein kinase enzyme inhibitor.[16]
Quercetin, in conjunction with dasatinib, is a senolytic; the combination is referred to as D+Q.[29]
Food safety
In 2010, the FDA acknowledged high-purity quercetin as GRAS for use as an ingredient in various specified food categories at levels up to 500 milligrams per serving.[30]
Health claims
Quercetin has been studied in basic research and small clinical trials.[2][31][32][33] While supplements have been promoted for the treatment of cancer and various other diseases,[2][34] there is no high-quality evidence that quercetin (via supplements or in food) is useful to treat cancer[35] or any other disease.[2][36]
The US Food and Drug Administration has issued warning letters to several manufacturers advertising on their product labels and websites that quercetin product(s) can be used to treat diseases.[37][38] The FDA regards such quercetin advertising and products as unapproved – with unauthorized health claims concerning the anti-disease products – as defined by "sections 201(g)(1)(B) and/or 201 (g)(1)(C) of the Act [21 U.S.C. § 321(g)(1)(B) and/or 21 U.S.C. § 321(g)(1)(C)] because they are intended for use in the diagnosis, cure, mitigation, treatment, or prevention of disease",[37][38] conditions not met by the manufacturers.
Safety
There has been little research into the safety of quercetin supplementation in humans, and the results are insufficient to give confidence that the practice is safe. In particular, there is a lack of safety information on the effect of quercetin supplementation for pregnant women, breastfeeding women, children, and adolescents. The hormonal effects of quercetin found in animal studies raise the suspicion of a parallel effect in humans, particularly in respect of estrogen-dependent tumors.[39]
Quercetin supplementation can interfere with the effects of medications. The precise nature of this interaction is known for some common medicines, but for many, it is not.[39]
See also
References
- "Quercetin dihydrate safety sheet". Archived from the original on September 16, 2011.
- "Flavonoids". Micronutrient Information Center, Linus Pauling Institute, Oregon State University, Corvallis, OR. November 2015. Retrieved 1 April 2018.
- "USDA Database for the Flavonoid Content of Selected Foods, Release 3" (PDF). U.S. Department of Agriculture. 2011.
- "Quercetin". Merriam-Webster.
- "Quercetin (biochemistry)". Encyclopædia Britannica.
- Fischer C, Speth V, Fleig-Eberenz S, Neuhaus G (Oct 1997). "Induction of Zygotic Polyembryos in Wheat: Influence of Auxin Polar Transport". The Plant Cell. 9 (10): 1767–1780. doi:10.1105/tpc.9.10.1767. PMC 157020. PMID 12237347.
- Formica JV, Regelson W (1995). "Review of the biology of quercetin and related bioflavonoids". Food and Chemical Toxicology. 33 (12): 1061–80. doi:10.1016/0278-6915(95)00077-1. PMID 8847003.
- Slimestad R, Fossen T, Vågen IM (December 2007). "Onions: a source of unique dietary flavonoids". Journal of Agricultural and Food Chemistry. 55 (25): 10067–80. doi:10.1021/jf0712503. PMID 17997520.
- Mitchell AE, Hong YJ, Koh E, Barrett DM, Bryant DE, Denison RF, Kaffka S (Jul 2007). "Ten-year comparison of the influence of organic and conventional crop management practices on the content of flavonoids in tomatoes". Journal of Agricultural and Food Chemistry. 55 (15): 6154–9. doi:10.1021/jf070344+. PMID 17590007.
- Petrus K, Schwartz H, Sontag G (Jun 2011). "Analysis of flavonoids in honey by HPLC coupled with coulometric electrode array detection and electrospray ionization mass spectrometry". Analytical and Bioanalytical Chemistry. 400 (8): 2555–63. doi:10.1007/s00216-010-4614-7. PMID 21229237. S2CID 24796542.
- Winkel-Shirley B (Jun 2001). "Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology". Plant Physiology. 126 (2): 485–93. doi:10.1104/pp.126.2.485. PMC 1540115. PMID 11402179.
- Juergenliemk G, Boje K, Huewel S, Lohmann C, Galla HJ, Nahrstedt A (Nov 2003). "In vitro studies indicate that miquelianin (quercetin 3-O-beta-D-glucuronopyranoside) is able to reach the CNS from the small intestine". Planta Medica. 69 (11): 1013–7. doi:10.1055/s-2003-45148. PMID 14735439. S2CID 260253046.
- "Information on EC 3.2.1.66 - quercitrinase". BRENDA (BRaunschweig ENzyme DAtabase). Helmholtz Centre for Infection Research.
- Tranchimand S, Brouant P, Iacazio G (Nov 2010). "The rutin catabolic pathway with special emphasis on quercetinase". Biodegradation. 21 (6): 833–59. doi:10.1007/s10532-010-9359-7. PMID 20419500. S2CID 30101803.
- Gugler, R.; Leschik, M.; Dengler, H. J. (1 March 1975). "Disposition of quercetin in man after single oral and intravenous doses". European Journal of Clinical Pharmacology. 9 (2): 229–234. doi:10.1007/BF00614022. PMID 1233267. S2CID 23812714.
- Williams RJ, Spencer JP, Rice-Evans C (Apr 2004). "Flavonoids: antioxidants or signalling molecules?". (review). Free Radical Biology & Medicine. 36 (7): 838–49. doi:10.1016/j.freeradbiomed.2004.01.001. PMID 15019969.
- Barnes S, Prasain J, D'Alessandro T, Arabshahi A, Botting N, Lila MA, Jackson G, Janle EM, Weaver CM (May 2011). "The metabolism and analysis of isoflavones and other dietary polyphenols in foods and biological systems". (review). Food & Function. 2 (5): 235–44. doi:10.1039/c1fo10025d. PMC 4122511. PMID 21779561.
- Luca SV, Macovei I, Bujor A, Trifan A (2020). "Bioactivity of dietary polyphenols: The role of metabolites". Critical Reviews in Food Science and Nutrition. 60 (4): 626–659. doi:10.1080/10408398.2018.1546669. PMID 30614249. S2CID 58651581.
- Dabeek WM, Marra MV (2019). "Dietary Quercetin and Kaempferol: Bioavailability and Potential Cardiovascular-Related Bioactivity in Humans". Nutrients. 11 (10): 2288. doi:10.3390/nu11102288. PMC 6835347. PMID 31557798.
- Graefe EU, Derendorf H, Veit M (1999). "Pharmacokinetics and bioavailability of the flavonol quercetin in humans" (PDF). (review). International Journal of Clinical Pharmacology and Therapeutics. 37 (5): 219–33. PMID 10363620. Archived from the original (PDF) on 2017-05-17. Retrieved 2016-01-01.
- Wittig, Jörg; Herderich, Markus; Graefe, Eva Ulrike; Veit, Markus (April 2001). "Identification of quercetin glucuronides in human plasma by high-performance liquid chromatography–tandem mass spectrometry". Journal of Chromatography B: Biomedical Sciences and Applications. 753 (2): 237–243. doi:10.1016/s0378-4347(00)00549-1. PMID 11334336.
- Day AJ, Rothwell JA, Morgan RA (2004). "Characterization of polyphenol metabolites". In Bao Y, Fenwick R (eds.). Phytochemicals in health and disease. New York, NY: Dekker. pp. 50–67. ISBN 0-8247-4023-8.
- Procházková D, Boušová I, Wilhelmová N (2011). "Antioxidant and prooxidant properties of flavonoids". Fitoterapia. 82 (4): 513–523. doi:10.1016/j.fitote.2011.01.018. PMID 21277359.
- Elbarbry F, Ung A, Abdelkawy K (January 2018). "Studying the Inhibitory Effect of Quercetin and Thymoquinone on Human Cytochrome P450 Enzyme Activities". Pharmacognosy Magazine. 13 (Suppl 4): S895–S899. doi:10.4103/0973-1296.224342 (inactive 1 August 2023). PMC 5822518. PMID 29491651.
{{cite journal}}: CS1 maint: DOI inactive as of August 2023 (link) - Rastogi, Himanshu; Jana, Snehasis (December 2014). "Evaluation of Inhibitory Effects of Caffeic acid and Quercetin on Human Liver Cytochrome P450 Activities". Phytotherapy Research. 28 (12): 1873–1878. doi:10.1002/ptr.5220. PMID 25196644. S2CID 41563915.
- Xiao, Jian; Huang, Wei-Hua; Peng, Jing-Bo; Tan, Zhi-Rong; Ou-Yang, Dong-Sheng; Hu, Dong-Li; Zhang, Wei; Chen, Yao (2014). "Quercetin Significantly Inhibits the Metabolism of Caffeine, a Substrate of Cytochrome P450 1A2 Unrelated to CYP1A2*1C (−2964G>A) and 1F* (734C>A) Gene Polymorphisms". BioMed Research International. 2014: 1–6. doi:10.1155/2014/405071. PMC 4082882. PMID 25025048.
- Hartman ML, Czyz M (2020). "BCL-w: apoptotic and non-apoptotic role in health and disease". Cell Death & Disease. 11 (4): 2260. doi:10.1038/s41419-020-2417-0. PMC 7174325. PMID 32317622.
- Paez-Ribes M, González-Gualda E, Doherty GJ, Muñoz-Espín D (2019). "Targeting senescent cells in translational medicine". EMBO Molecular Medicine. 11 (12): e10234. doi:10.15252/emmm.201810234. PMC 6895604. PMID 31746100.
- Kirkland JL, Tchkonia T (2020). "Senolytic drugs: from discovery to translation". Journal of Internal Medicine. 288 (5): 518–536. doi:10.1111/joim.13141. PMC 7405395. PMID 32686219.
- "GRN No. 341 (Quercetin)". US Food and Drug Administration. 22 November 2010. Retrieved 27 October 2021.
- Yang F, Song L, Wang H, Wang J, Xu Z, Xing N (June 2015). "Quercetin in prostate cancer: Chemotherapeutic and chemopreventive effects, mechanisms and clinical application potential (Review)". Oncol. Rep. 33 (6): 2659–68. doi:10.3892/or.2015.3886. PMID 25845380.
- Gross P (March 1, 2009), New Roles for Polyphenols. A 3-Part Report on Current Regulations & the State of Science, Nutraceuticals World
- Miles SL, McFarland M, Niles RM (2014). "Molecular and physiological actions of quercetin: need for clinical trials to assess its benefits in human disease". Nutrition Reviews. 72 (11): 720–34. doi:10.1111/nure.12152. PMID 25323953.
- D'Andrea G (2015). "Quercetin: A flavonol with multifaceted therapeutic applications?". Fitoterapia. 106: 256–71. doi:10.1016/j.fitote.2015.09.018. PMID 26393898.
- Ades TB, ed. (2009). Quercetin. ISBN 9780944235713.
{{cite book}}:|work=ignored (help) - European Food Safety Agency (EFSA) NDA Panel (Dietetic Products, Nutrition and Allergies) (8 April 2011). "Scientific Opinion on the substantiation of health claims related to quercetin and protection of DNA, proteins and lipids from oxidative damage (ID 1647), "cardiovascular system" (ID 1844), "mental state and performance" (ID 1845), and "liver, kidneys" (ID 1846) pursuant to Article 13(1) of Regulation (EC) No 1924/2006". EFSA Journal. 9 (4): 2067–82. doi:10.2903/j.efsa.2011.2067. Retrieved 24 September 2014.
- King JL (2 March 2017). "Warning Letter to Cape Fear Naturals". Inspections, Compliance, Enforcement, and Criminal Investigations, US Food and Drug Administration. Retrieved 29 November 2018.
- Pace R (17 April 2017). "Warning Letter to DoctorVicks.com". Inspections, Compliance, Enforcement, and Criminal Investigations, US Food and Drug Administration. Retrieved 29 November 2018.
- Andres S, Pevny S, Ziegenhagen R, Bakhiya N, Schäfer B, Hirsch-Ernst KI, Lampen A (January 2018). "Safety Aspects of the Use of Quercetin as a Dietary Supplement". Mol Nutr Food Res (Review). 62 (1). doi:10.1002/mnfr.201700447. PMID 29127724. S2CID 24772872.
External links
 Media related to Quercetin at Wikimedia Commons
Media related to Quercetin at Wikimedia Commons