Decynium-22
Decynium-22 is a cationic derivative of quinoline, and a potent inhibitor of the plasma membrane monoamine transporter (PMAT), as well as all members of the organic cation transporter (OCT) family in both human and rat cells.[1] However, it has little effect on high affinity monoamine transporters such as the dopamine transporter and norepinephrine transporter.[2]
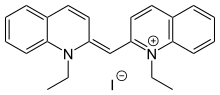 | |
| Names | |
|---|---|
| Preferred IUPAC name
1-Ethyl-2-[(E)-(1-ethylquinolin-2(1H)-ylidene)methyl]quinolin-1-ium iodide | |
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.012.324 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C23H23IN2 | |
| Molar mass | 454.355 g·mol−1 |
| Appearance | Dark red powder |
| Melting point | 273 °C (523 °F; 546 K) |
| Solubility | 4.54 mg/ml in DMSO |
| Hazards | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Transporter inhibition
Decynium has been shown to have a very high affinity to organic cation transporters in a variety of species, including human,[3][4] rat,[5] and pig.[6] Decynium-22 has been shown to block the uptake of the neurotoxin 1-methyl-4-phenylpyridinium (MPP) via the OCT3 transporter in rat astrocytes.[7]
Fluorescence
Decynium-22 emits a low fluorescence yield (around 0.001), and in its monomeric form is a weakly fluorescent. However, aggregated decynium-22 emits a strong superradiant emission with a maximum near 570–580 nm.[8][9] 480 nm light falls near a short wavelength peak of the excitation spectrum of these aggregates. Decynium-22 fluorescence caused by aggregation can be observed in astrocytes.[2]
Schizophrenia and depression
Decynium-22 has recently been investigated for its role in increasing extracellular serotonin in the brain in neuropharmacology research. The transportation of the neurotransmitter serotonin is often disrupted in psychiatric disorders characterized by social impairment, such as schizophrenia and depression. Serotonin is primarily taken up by the 5-HT transporter (SERT), although it is also taken up by auxiliary transporters, known as "uptake 2", which include OCT and PMAT.
The most commonly prescribed antidepressant drugs are the selective serotonin reuptake inhibitors (SSRIs), which act by blocking the high affinity SERT. A proposed explanation for the limited efficacy of SSRIs is the presence of the low affinity transporters OCT and PMAT, which limit the ability of SSRIs to increase extracellular serotonin. Decynium-22 blocked serotonin uptake via these auxiliary transporters, and when used in conjunction with SSRIs, decynium-22 enhanced the effects of SSRIs to inhibit serotonin clearance.[10] A similar effect was seen in SERT knock-out mice, which resulted in an improvement of social behavior.[11] When OCT3 was knocked out in mice, however, decynium-22 was ineffectual, indicating that the anti-depressant effects of decynium-22 are dependent upon its blockage of the OCT3.[10]
References
- "Decynium-22". University of California, San Francisco. Archived from the original on 17 October 2014. Retrieved 16 Oct 2014.
- Inyushin M, Kucheryaykh Y, Kucheryavykh L, Sanabria P, Jiménez-Rivera C, Struganova I, et al. (2010). "Membrane potential and pH-dependent accumulation of decynium-22 (1,1′-diethyl-2,2′-cyanine iodide) fluorescence through the low affinity OCT transporters in astrocytes". Bol Asoc Med P R. 102 (3): 5–12. PMC 3721433. PMID 23875515.
- Russ H, Sonna J, Keppler K, Baunach S, Schömig E (1993). "Cyanine-related compounds: a novel class of potent inhibitors of extraneuronal noradrenaline transport". Naunyn Schmiedebergs Arch Pharmacol. 348 (5): 458–65. doi:10.1007/bf00173203. PMID 8114944. S2CID 6093183.
- Hayer-Zillgen M, Brüss M, Bönisch H (2002). "Expression and pharmacological profile of the human organic cation transporters hOCT1, hOCT2 and hOCT3". Br J Pharmacol. 136 (6): 829–36. doi:10.1038/sj.bjp.0704785. PMC 1573414. PMID 12110607.
- Gründemann D, Gorboulev V, Gambaryan S, Veyhl M, Koepsell H (1994). "Drug excretion mediated by a new prototype of polyspecific transporter". Nature. 372 (6506): 549–552. Bibcode:1994Natur.372..549G. doi:10.1038/372549a0. PMID 7990927. S2CID 659539.
- Gründemann D, Babin-Ebell J, Martel F, Ording N, Schmidt A, Schömig E (1997). "Primary structure and functional expression of the apical organic cation transporter from kidney epithelial LLC-PK1 cells". J Biol Chem. 272 (16): 10408–13. doi:10.1074/jbc.272.16.10408. PMID 9099681.
- Inazu M, Takeda H, Matsumiya T (2003). "Expression and functional characterization of the extraneuronal monoamine transporter in normal human astrocytes". J Neurochem. 84 (1): 43–52. doi:10.1046/j.1471-4159.2003.01566.x. PMID 12485400.
- Stiel H, Daehne S, Teuchner K. J-aggregates of pseudoisocyanine in solution: New data from nonlinear spectroscopy. J Lumines. 1988;39:351–357.
- Struganova IA. Dynamics of formation of 1,1′-diethyl-2,2′-cyanine iodide J-aggregates in solution. J Physical Chem A. 2000;104:9670–9674.
- Horton RE, Apple DM, Owens WA, Baganz NL, Cano S, Mitchell NC, et al. (2013). "Decynium-22 enhances SSRI-induced antidepressant-like effects in mice: uncovering novel targets to treat depression". J Neurosci. 33 (25): 10534–43. doi:10.1523/JNEUROSCI.5687-11.2013. PMC 3685842. PMID 23785165.
- Loginova LG, Iakovleva MB, Golovina IG, Bogdanova TI (1976). "[Yeast cell wall-dissolving enzymes of the thermotolerant actinomycete Thermoactinomyces vulgaris]". Mikrobiologiia. 45 (2): 291–7. PMID 6860.
