Carbohydrate-binding module
In molecular biology, a carbohydrate-binding module (CBM) is a protein domain found in carbohydrate-active enzymes (for example glycoside hydrolases). The majority of these domains have carbohydrate-binding activity. Some of these domains are found on cellulosomal scaffoldin proteins. CBMs were previously known as cellulose-binding domains.[1] CBMs are classified into numerous families, based on amino acid sequence similarity. There are currently (June 2011) 64 families of CBM in the CAZy database.[2]
| CBM_1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 three-dimensional structures of three engineered cellulose-binding domains of cellobiohydrolase i from trichoderma reesei, nmr, 18 structures | |||||||||
| Identifiers | |||||||||
| Symbol | CBM_1 | ||||||||
| Pfam | PF00734 | ||||||||
| InterPro | IPR000254 | ||||||||
| PROSITE | PDOC00486 | ||||||||
| SCOP2 | 1cel / SCOPe / SUPFAM | ||||||||
| CAZy | CBM1 | ||||||||
| |||||||||
| CBM_2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 solution structure of a cellulose binding domain from cellulomonas fimi by nuclear magnetic resonance spectroscopy | |||||||||
| Identifiers | |||||||||
| Symbol | CBM_2 | ||||||||
| Pfam | PF00553 | ||||||||
| Pfam clan | CL0203 | ||||||||
| InterPro | IPR001919 | ||||||||
| PROSITE | PDOC00485 | ||||||||
| SCOP2 | 1exg / SCOPe / SUPFAM | ||||||||
| CAZy | CBM2 | ||||||||
| |||||||||
| CBM_3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 crystal structure of a family iiia cbd from clostridium cellulolyticum | |||||||||
| Identifiers | |||||||||
| Symbol | CBM_3 | ||||||||
| Pfam | PF00942 | ||||||||
| Pfam clan | CL0203 | ||||||||
| InterPro | IPR001956 | ||||||||
| SCOP2 | 1nbc / SCOPe / SUPFAM | ||||||||
| CAZy | CBM3 | ||||||||
| |||||||||
| CBM_5/12 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 interactions of a family 18 chitinase with the designed inhibitor hm508, and its degradation product, chitobiono-delta-lactone | |||||||||
| Identifiers | |||||||||
| Symbol | CBM_5_12 | ||||||||
| Pfam | PF02839 | ||||||||
| InterPro | IPR003610 | ||||||||
| SCOP2 | 1ed7 / SCOPe / SUPFAM | ||||||||
| CAZy | CBM12 | ||||||||
| |||||||||
| CBM_6 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
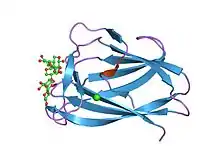 cbm6ct from clostridium thermocellum in complex with xylopentaose | |||||||||
| Identifiers | |||||||||
| Symbol | CBM_6 | ||||||||
| Pfam | PF03422 | ||||||||
| Pfam clan | CL0202 | ||||||||
| InterPro | IPR005084 | ||||||||
| SCOP2 | 1gmm / SCOPe / SUPFAM | ||||||||
| CAZy | CBM6 | ||||||||
| |||||||||
| CBM_4/9 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
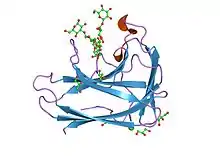 cbm4 structure and function | |||||||||
| Identifiers | |||||||||
| Symbol | CBM_4_9 | ||||||||
| Pfam | PF02018 | ||||||||
| Pfam clan | CL0202 | ||||||||
| InterPro | IPR003305 | ||||||||
| SCOP2 | 1ulp / SCOPe / SUPFAM | ||||||||
| CAZy | CBM22 | ||||||||
| |||||||||
| CBM_10 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 solution structure of type x cbm | |||||||||
| Identifiers | |||||||||
| Symbol | CBM_10 | ||||||||
| Pfam | PF02013 | ||||||||
| InterPro | IPR002883 | ||||||||
| SCOP2 | 1qld / SCOPe / SUPFAM | ||||||||
| CAZy | CBM10 | ||||||||
| |||||||||
| CBM_11 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
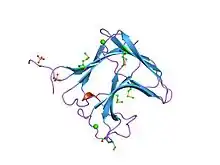 family 11 carbohydrate-binding module of cellulosomal cellulase lic26a-cel5e of clostridium thermocellum | |||||||||
| Identifiers | |||||||||
| Symbol | CBM_11 | ||||||||
| Pfam | PF03425 | ||||||||
| Pfam clan | CL0202 | ||||||||
| InterPro | IPR005087 | ||||||||
| CAZy | CBM11 | ||||||||
| |||||||||
| CBM_14 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | CBM_14 | ||||||||
| Pfam | PF01607 | ||||||||
| Pfam clan | CL0155 | ||||||||
| InterPro | IPR002557 | ||||||||
| SCOP2 | 1dqc / SCOPe / SUPFAM | ||||||||
| CAZy | CBM14 | ||||||||
| |||||||||
| CBM_15 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
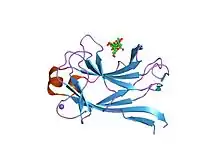 xylan-binding module cbm15 | |||||||||
| Identifiers | |||||||||
| Symbol | CBM_15 | ||||||||
| Pfam | PF03426 | ||||||||
| Pfam clan | CL0202 | ||||||||
| InterPro | IPR005088 | ||||||||
| SCOP2 | 1gny / SCOPe / SUPFAM | ||||||||
| CAZy | CBM15 | ||||||||
| |||||||||
| CBM_17/28 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 structure of fam17 carbohydrate binding module from clostridium cellulovorans | |||||||||
| Identifiers | |||||||||
| Symbol | CBM_17_28 | ||||||||
| Pfam | PF03424 | ||||||||
| Pfam clan | CL0202 | ||||||||
| InterPro | IPR005086 | ||||||||
| SCOP2 | 1g0c / SCOPe / SUPFAM | ||||||||
| CAZy | CBM28 | ||||||||
| |||||||||
| Chitin_bind_1 (CBM18) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 crystal structure analysis of crosslinked-wga3/glcnacbeta1,4glcnac complex | |||||||||
| Identifiers | |||||||||
| Symbol | Chitin_bind_1 | ||||||||
| Pfam | PF00187 | ||||||||
| InterPro | IPR001002 | ||||||||
| PROSITE | PDOC00025 | ||||||||
| SCOP2 | 1wgt / SCOPe / SUPFAM | ||||||||
| CAZy | CBM18 | ||||||||
| |||||||||
| CBM_19 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | CBM_19 | ||||||||
| Pfam | PF03427 | ||||||||
| Pfam clan | CL0155 | ||||||||
| InterPro | IPR005089 | ||||||||
| CAZy | CBM19 | ||||||||
| |||||||||
| CBM_20 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 glucoamylase, granular starch-binding domain complex with cyclodextrin, nmr, minimized average structure | |||||||||
| Identifiers | |||||||||
| Symbol | CBM_20 | ||||||||
| Pfam | PF00686 | ||||||||
| Pfam clan | CL0369 | ||||||||
| InterPro | IPR002044 | ||||||||
| SCOP2 | 1cdg / SCOPe / SUPFAM | ||||||||
| CAZy | CBM20 | ||||||||
| |||||||||
| CBM_21 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | CBM_21 | ||||||||
| Pfam | PF03370 | ||||||||
| InterPro | IPR005036 | ||||||||
| CAZy | CBM21 | ||||||||
| |||||||||
| CBM_25 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | CBM_25 | ||||||||
| Pfam | PF03423 | ||||||||
| InterPro | IPR005085 | ||||||||
| CAZy | CBM25 | ||||||||
| |||||||||
| CBM27 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 structural and thermodynamic dissection of specific mannan recognition by a carbohydrate-binding module, tmcbm27 | |||||||||
| Identifiers | |||||||||
| Symbol | CBM27 | ||||||||
| Pfam | PF09212 | ||||||||
| InterPro | IPR015295 | ||||||||
| SCOP2 | 1oh4 / SCOPe / SUPFAM | ||||||||
| |||||||||
| Chitin_bind_3 (CBM33) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 crystal structure of the serratia marcescens chitin-binding protein cbp21 y54a mutant. | |||||||||
| Identifiers | |||||||||
| Symbol | Chitin_bind_3 | ||||||||
| Pfam | PF03067 | ||||||||
| InterPro | IPR004302 | ||||||||
| CAZy | CBM33 | ||||||||
| |||||||||
| CBM_48 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 crystal structure of glycosyltrehalose trehalohydrolase from sulfolobus solfataricus | |||||||||
| Identifiers | |||||||||
| Symbol | CBM_48 | ||||||||
| Pfam | PF02922 | ||||||||
| Pfam clan | CL0369 | ||||||||
| InterPro | IPR004193 | ||||||||
| SCOP2 | 1bf2 / SCOPe / SUPFAM | ||||||||
| CAZy | CBM48 | ||||||||
| |||||||||
| CBM49 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | CBM49 | ||||||||
| Pfam | PF09478 | ||||||||
| Pfam clan | CL0203 | ||||||||
| InterPro | IPR019028 | ||||||||
| |||||||||
CBMs of microbial glycoside hydrolases play a central role in the recycling of photosynthetically fixed carbon through their binding to specific plant structural polysaccharides.[3] CBMs can recognise both crystalline and amorphous cellulose forms.[4] CBMs are the most common non-catalytic modules associated with enzymes active in plant cell-wall hydrolysis. Many putative CBMs have been identified by amino acid sequence alignments but only a few representatives have been shown experimentally to have a carbohydrate-binding function.[5]
CBM1
Carbohydrate-binding module family 1 (CBM1) consists of 36 amino acids. This domain contains 4 conserved cysteine residues which are involved in the formation of two disulfide bonds.
CBM2
Carbohydrate-binding module family 2 (CBM2) contains two conserved cysteines - one at each extremity of the domain - which have been shown [6] to be involved in a disulfide bond. There are also four conserved tryptophans, two of which are involved in cellulose binding.[7][8][9]
CBM3
Carbohydrate-binding module family 3 (CBM3) is involved in cellulose binding [10] and is found associated with a wide range of bacterial glycosyl hydrolases. The structure of this domain is known; it forms a beta sandwich.[11]
CBM4
Carbohydrate-binding module family 4 (CBM4) includes the two cellulose-binding domains, CBD(N1) and CBD(N2), arranged in tandem at the N terminus of the 1,4-beta-glucanase, CenC, from Cellulomonas fimi. These homologous CBMs are distinct in their selectivity for binding amorphous and not crystalline cellulose.[12] Multidimensional heteronuclear nuclear magnetic resonance (NMR) spectroscopy was used to determine the tertiary structure of the 152 amino acid N-terminal cellulose-binding domain from C. fimi 1,4-beta-glucanase CenC (CBDN1). The tertiary structure of CBDN1 is strikingly similar to that of the bacterial 1,3-1,4-beta-glucanases, as well as other sugar-binding proteins with jelly-roll folds.[13] CBM4 and CBM9 are closely related.
CBM5
Carbohydrate-binding module family 5 (CBM5) binds chitin.[14] CBM5 and CBM12 are distantly related.
CBM6
Carbohydrate-binding module family 6 (CBM6) is unusual in that it contains two substrate-binding sites, cleft A and cleft B. Cellvibrio mixtus endoglucanase 5A contains two CBM6 domains, the CBM6 domain at the C-terminus displays distinct ligand binding specificities in each of the substrate-binding clefts. Both cleft A and cleft B can bind cello-oligosaccharides, laminarin preferentially binds in cleft A, xylooligosaccharides only bind in cleft A and beta1,4,-beta1,3-mixed linked glucans only bind in cleft B.[15]
CBM9
Carbohydrate-binding module family 9 (CBM9) binds to crystalline cellulose.[16] CBM4 and CBM9 are closely related.
CBM10
Carbohydrate-binding module family 10 (CBM10) is found in two distinct sets of proteins with different functions. Those found in aerobic bacteria bind cellulose (or other carbohydrates); but in anaerobic fungi they are protein binding domains, referred to as dockerin domains. The dockerin domains are believed to be responsible for the assembly of a multiprotein cellulase/hemicellulase complex, similar to the cellulosome found in certain anaerobic bacteria.[17][18]
In anaerobic bacteria that degrade plant cell walls, exemplified by Clostridium thermocellum, the dockerin domains of the catalytic polypeptides can bind equally well to any cohesin from the same organism. More recently, anaerobic fungi, typified by Piromyces equi, have been suggested to also synthesise a cellulosome complex, although the dockerin sequences of the bacterial and fungal enzymes are completely different.[19] For example, the fungal enzymes contain one, two or three copies of the dockerin sequence in tandem within the catalytic polypeptide. In contrast, all the C. thermocellum cellulosome catalytic components contain a single dockerin domain. The anaerobic bacterial dockerins are homologous to EF hands (calcium-binding motifs) and require calcium for activity whereas the fungal dockerin does not require calcium. Finally, the interaction between cohesin and dockerin appears to be species specific in bacteria, there is almost no species specificity of binding within fungal species and no identified sites that distinguish different species.
The of dockerin from P. equi contains two helical stretches and four short beta-strands which form an antiparallel sheet structure adjacent to an additional short twisted parallel strand. The N- and C-termini are adjacent to each other.[19]
CBM11
Carbohydrate-binding module family 11 (CBM11) is found in a number of bacterial cellulases. One example is the CBM11 of Clostridium thermocellum Cel26A-Cel5E, this domain has been shown to bind both β-1,4-glucan and β-1,3-1,4-mixed linked glucans.[20] CBM11 has beta-sandwich structure with a concave side forming a substrate-binding cleft.[20]
CBM12
Carbohydrate-binding module family 12 (CBM12) comprises two beta-sheets, consisting of two and three antiparallel beta strands respectively. It binds chitin via the aromatic rings of tryptophan residues.[14] CBM5 and CBM12 are distantly related.
CBM14
Carbohydrate-binding module family 14 (CBM14) is also known as the peritrophin-A domain. It is found in chitin binding proteins, particularly the peritrophic matrix proteins of insects and animal chitinases.[21][22][23] Copies of the domain are also found in some baculoviruses. It is an extracellular domain that contains six conserved cysteines that probably form three disulfide bridges. Chitin binding has been demonstrated for a protein containing only two of these domains.[21]
CBM15
Carbohydrate-binding module family 15 (CBM15), found in bacterial enzymes, has been shown to bind to xylan and xylooligosaccharides. It has a beta-jelly roll fold, with a groove on the concave surface of one of the beta-sheets.[3]
CBM17
Carbohydrate-binding module family 17 (CBM17) appears to have a very shallow binding cleft that may be more accessible to cellulose chains in non-crystalline cellulose than the deeper binding clefts of family 4 CBMs.[24] Sequence and structural conservation in families CBM17 and CBM28 suggests that they have evolved through gene duplication and subsequent divergence.[4] CBM17 does not compete with CBM28 modules when binding to non-crystalline cellulose. Different CBMs have been shown to bind to different sites in amorphous cellulose, CBM17 and CBM28 recognise distinct non-overlapping sites in amorphous cellulose.[25]
CBM18
Carbohydrate-binding module family 18 (CBM18) (also known as chitin binding 1 or chitin recognition protein) is found in a number of plant and fungal proteins that bind N-acetylglucosamine (e.g. solanaceous lectins of tomato and potato, plant endochitinases, the wound-induced proteins: hevein, win1 and win2, and the Kluyveromyces lactis killer toxin alpha subunit).[26] The domain may occur in one or more copies and is thought to be involved in recognition or binding of chitin subunits.[27][28] In chitinases, as well as in the potato wound-induced proteins, this 43-residue domain directly follows the signal sequence and is therefore at the N terminus of the mature protein; in the killer toxin alpha subunit it is located in the central section of the protein.
CBM19
Carbohydrate-binding module family 19 (CBM19), found in fungal chitinases, binds chitin.[29]
CBM21
Carbohydrate-binding module family 21 (CBM21), found in many eukaryotic proteins involved in glycogen metabolism, binds to glycogen.[32]
CBM25
Carbohydrate-binding module family 25 (CBM25) binds alpha-glucooligosaccharides, particularly those containing alpha-1,6 linkages, and granular starch.[33]
CBM27
Carbohydrate-binding module family 27 (CBM27) binds to beta-1,4-mannooligosaccharides, carob galactomannan, and konjac glucomannan, but not to cellulose (insoluble and soluble) or soluble birchwood xylan. CBM27 adopts a beta sandwich structure comprising 13 beta strands with a single, small alpha-helix and a single metal atom.[34]
CBM28
Carbohydrate-binding module family 28 (CBM28) does not compete with CBM17 modules when binding to non-crystalline cellulose. Different CBMs have been shown to bind to different sirtes in amorphous cellulose, CBM17 and CBM28 recognise distinct non-overlapping sites in amorphous cellulose. CBM28 has a "beta-jelly roll" topology, which is similar in structure to the CBM17 domains. Sequence and structural conservation in families CBM17 and CBM28 suggests that they have evolved through gene duplication and subsequent divergence.[4][25]
CBM32
Carbohydrate-binding module family 32 (CBM32) binds to diverse substrates, ranging from plant cell wall polysaccharides to complex glycans.[35] The module has so far been found in microorganisms, including archea, eubacteria and fungi.[35] CBM32 adopts a beta-sandwich fold and has a bound metal atom, most often observed to be calcium.[36] CBM32 modules are associated with catalytic modules such as sialidases, B-N-acetylglucosaminidases, α-N-acetylglucosaminidases, mannanases and galactose oxidases.[36]
CBM33
Carbohydrate-binding module family 33 (CBM33) is a chitin-binding domain.[37] It has a budded fibronectin type III fold consisting of two beta-sheets, arranged as a beta-sheet sandwich and a bud consisting of three short helices, located between beta-strands 1 and 2. It binds chitin via conserved polar amino acids.[38] This domain is found in isolation in baculoviral spheroidin and spindolin proteins.
CBM48
Carbohydrate-binding module family 48 (CBM48) is often found in enzymes containing glycosyl hydrolase family 13 catalytic domains. It is found in a range of enzymes that act on branched substrates i.e. isoamylase, pullulanase and branching enzyme. Isoamylase hydrolyses 1,6-alpha-D-glucosidic branch linkages in glycogen, amylopectin and dextrin; 1,4-alpha-glucan branching enzyme functions in the formation of 1,6-glucosidic linkages of glycogen; and pullulanase is a starch-debranching enzyme. CBM48 binds glycogen.[39][40][41][42]
CBM49
Carbohydrate-binding module family 49 (CBM49) is found at the C-terminal of cellulases and in vitro binding studies have shown it to binds to crystalline cellulose.[43]
References
- Gilkes NR, Henrissat B, Kilburn DG, Miller RC, Warren RA (June 1991). "Domains in microbial beta-1, 4-glycanases: sequence conservation, function, and enzyme families". Microbiol. Rev. 55 (2): 303–15. doi:10.1128/MMBR.55.2.303-315.1991. PMC 372816. PMID 1886523.
- Cantarel, B. L.; Coutinho, P. M.; Rancurel, C.; Bernard, T.; Lombard, V.; Henrissat, B. (2009). "The Carbohydrate-Active EnZymes database (CAZy): An expert resource for Glycogenomics". Nucleic Acids Research. 37 (Database issue): D233–D238. doi:10.1093/nar/gkn663. PMC 2686590. PMID 18838391.
- Szabo, L.; Jamal, S.; Xie, H.; Charnock, S. J.; Bolam, D. N.; Gilbert, H. J.; Davies, G. J. (2001). "Structure of a Family 15 Carbohydrate-binding Module in Complex with Xylopentaose. Evidence that xylan binds in an approximate 3-fold helical conformation". Journal of Biological Chemistry. 276 (52): 49061–49065. doi:10.1074/jbc.M109558200. PMID 11598143.
- Jamal S, Nurizzo D, Boraston AB, Davies GJ (May 2004). "X-ray crystal structure of a non-crystalline cellulose-specific carbohydrate-binding module: CBM28". J. Mol. Biol. 339 (2): 253–8. doi:10.1016/j.jmb.2004.03.069. PMID 15136030.
- Roske Y, Sunna A, Pfeil W, Heinemann U (July 2004). "High-resolution crystal structures of Caldicellulosiruptor strain Rt8B.4 carbohydrate-binding module CBM27-1 and its complex with mannohexaose". J. Mol. Biol. 340 (3): 543–54. doi:10.1016/j.jmb.2004.04.072. PMID 15210353.
- Gilkes NR, Claeyssens M, Aebersold R, Henrissat B, Meinke A, Morrison HD, Kilburn DG, Warren RA, Miller RC (December 1991). "Structural and functional relationships in two families of beta-1,4-glycanases". Eur. J. Biochem. 202 (2): 367–77. doi:10.1111/j.1432-1033.1991.tb16384.x. PMID 1761039.
- Meinke A, Gilkes NR, Kilburn DG, Miller RC, Warren RA (December 1991). "Bacterial cellulose-binding domain-like sequences in eucaryotic polypeptides". Protein Seq. Data Anal. 4 (6): 349–53. PMID 1812490.
- Simpson PJ, Xie H, Bolam DN, Gilbert HJ, Williamson MP (December 2000). "The structural basis for the ligand specificity of family 2 carbohydrate-binding modules". J. Biol. Chem. 275 (52): 41137–42. doi:10.1074/jbc.M006948200. PMID 10973978.
- Xu, G. Y.; Ong, E.; Gilkes, N. R.; Kilburn, D. G.; Muhandiram, D. R.; Harris-Brandts, M.; Carver, J. P.; Kay, L. E.; Harvey, T. S. (1995). "Solution structure of a cellulose-binding domain from Cellulomonas fimi by nuclear magnetic resonance spectroscopy". Biochemistry. 34 (21): 6993–7009. doi:10.1021/bi00021a011. PMID 7766609.
- Poole DM, Morag E, Lamed R, Bayer EA, Hazlewood GP, Gilbert HJ (December 1992). "Identification of the cellulose-binding domain of the cellulosome subunit S1 from Clostridium thermocellum YS". FEMS Microbiol. Lett. 78 (2–3): 181–6. doi:10.1016/0378-1097(92)90022-g. PMID 1490597.
- Tormo J, Lamed R, Chirino AJ, Morag E, Bayer EA, Shoham Y, Steitz TA (November 1996). "Crystal structure of a bacterial family-III cellulose-binding domain: a general mechanism for attachment to cellulose". EMBO J. 15 (21): 5739–51. doi:10.1002/j.1460-2075.1996.tb00960.x. PMC 452321. PMID 8918451.
- Brun E, Johnson PE, Creagh AL, Tomme P, Webster P, Haynes CA, McIntosh LP (March 2000). "Structure and binding specificity of the second N-terminal cellulose-binding domain from Cellulomonas fimi endoglucanase C". Biochemistry. 39 (10): 2445–58. doi:10.1021/bi992079u. PMID 10704194.
- Johnson PE, Joshi MD, Tomme P, Kilburn DG, McIntosh LP (November 1996). "Structure of the N-terminal cellulose-binding domain of Cellulomonas fimi CenC determined by nuclear magnetic resonance spectroscopy". Biochemistry. 35 (45): 14381–94. doi:10.1021/bi961612s. PMID 8916925.
- Akagi, K. -I.; Watanabe, J.; Hara, M.; Kezuka, Y.; Chikaishi, E.; Yamaguchi, T.; Akutsu, H.; Nonaka, T.; Watanabe, T.; Ikegami, T. (2006). "Identification of the Substrate Interaction Region of the Chitin-Binding Domain of Streptomyces griseus Chitinase C". Journal of Biochemistry. 139 (3): 483–493. doi:10.1093/jb/mvj062. PMID 16567413.
- Henshaw, J. L.; Bolam, D. N.; Pires, V. M.; Czjzek, M.; Henrissat, B.; Ferreira, L. M.; Fontes, C. M.; Gilbert, H. J. (2004). "The Family 6 Carbohydrate Binding Module CmCBM6-2 Contains Two Ligand-binding Sites with Distinct Specificities". Journal of Biological Chemistry. 279 (20): 21552–21559. doi:10.1074/jbc.M401620200. PMID 15004011.
- Winterhalter, C.; Heinrich, P.; Candussio, A.; Wich, G.; Liebl, W. (1995). "Identification of a novel cellulose-binding domain within the multidomain 120 kDa xylanase XynA of the hyperthermophilic bacterium Thermotoga maritima". Molecular Microbiology. 15 (3): 431–444. doi:10.1111/j.1365-2958.1995.tb02257.x. PMID 7783614. S2CID 25985173.
- Millward-Sadler SJ, Davidson K, Hazlewood GP, Black GW, Gilbert HJ, Clarke JH (November 1995). "Novel cellulose-binding domains, NodB homologues and conserved modular architecture in xylanases from the aerobic soil bacteria Pseudomonas fluorescens subsp. cellulosa and Cellvibrio mixtus". Biochem. J. 312 (1): 39–48. doi:10.1042/bj3120039. PMC 1136224. PMID 7492333.
- Fanutti C, Ponyi T, Black GW, Hazlewood GP, Gilbert HJ (December 1995). "The conserved noncatalytic 40-residue sequence in cellulases and hemicellulases from anaerobic fungi functions as a protein docking domain". J. Biol. Chem. 270 (49): 29314–22. doi:10.1074/jbc.270.49.29314. PMID 7493964.
- Raghothama S, Eberhardt RY, Simpson P, Wigelsworth D, White P, Hazlewood GP, Nagy T, Gilbert HJ, Williamson MP (September 2001). "Characterization of a cellulosome dockerin domain from the anaerobic fungus Piromyces equi". Nat. Struct. Biol. 8 (9): 775–8. doi:10.1038/nsb0901-775. PMID 11524680. S2CID 6442375.
- Carvalho, A. L.; Goyal, A.; Prates, J. A.; Bolam, D. N.; Gilbert, H. J.; Pires, V. M.; Ferreira, L. M.; Planas, A.; Romão, M. J.; Fontes, C. M. (2004). "The Family 11 Carbohydrate-binding Module of Clostridium thermocellum Lic26A-Cel5E Accommodates -1,4- and -1,3-1,4-Mixed Linked Glucans at a Single Binding Site". Journal of Biological Chemistry. 279 (33): 34785–34793. doi:10.1074/jbc.M405867200. PMID 15192099.
- Shen Z, Jacobs-Lorena M (July 1998). "A type I peritrophic matrix protein from the malaria vector Anopheles gambiae binds to chitin. Cloning, expression, and characterization". J. Biol. Chem. 273 (28): 17665–70. doi:10.1074/jbc.273.28.17665. PMID 9651363.
- Elvin CM, Vuocolo T, Pearson RD, East IJ, Riding GA, Eisemann CH, Tellam RL (April 1996). "Characterization of a major peritrophic membrane protein, peritrophin-44, from the larvae of Lucilia cuprina. cDNA and deduced amino acid sequences". J. Biol. Chem. 271 (15): 8925–35. doi:10.1074/jbc.271.15.8925. PMID 8621536.
- Casu R, Eisemann C, Pearson R, Riding G, East I, Donaldson A, Cadogan L, Tellam R (August 1997). "Antibody-mediated inhibition of the growth of larvae from an insect causing cutaneous myiasis in a mammalian host". Proc. Natl. Acad. Sci. U.S.A. 94 (17): 8939–44. Bibcode:1997PNAS...94.8939C. doi:10.1073/pnas.94.17.8939. PMC 22971. PMID 9256413.
- Notenboom V, Boraston AB, Chiu P, Freelove AC, Kilburn DG, Rose DR (December 2001). "Recognition of cello-oligosaccharides by a family 17 carbohydrate-binding module: an X-ray crystallographic, thermodynamic and mutagenic study". J. Mol. Biol. 314 (4): 797–806. doi:10.1006/jmbi.2001.5153. PMID 11733998.
- Jamal, S.; Nurizzo, D.; Boraston, A. B.; Davies, G. J. (2004). "X-ray Crystal Structure of a Non-crystalline Cellulose-specific Carbohydrate-binding Module: CBM28". Journal of Molecular Biology. 339 (2): 253–258. doi:10.1016/j.jmb.2004.03.069. PMID 15136030.
- Wright HT, Sandrasegaram G, Wright CS (September 1991). "Evolution of a family of N-acetylglucosamine binding proteins containing the disulfide-rich domain of wheat germ agglutinin". J. Mol. Evol. 33 (3): 283–94. Bibcode:1991JMolE..33..283W. doi:10.1007/bf02100680. PMID 1757999. S2CID 8327744.
- Butler AR, O'Donnell RW, Martin VJ, Gooday GW, Stark MJ (July 1991). "Kluyveromyces lactis toxin has an essential chitinase activity". Eur. J. Biochem. 199 (2): 483–8. doi:10.1111/j.1432-1033.1991.tb16147.x. PMID 2070799.
- Lerner DR, Raikhel NV (June 1992). "The gene for stinging nettle lectin (Urtica dioica agglutinin) encodes both a lectin and a chitinase". J. Biol. Chem. 267 (16): 11085–91. doi:10.1016/S0021-9258(19)49878-5. PMID 1375935.
- Kuranda, M. J.; Robbins, P. W. (1991). "Chitinase is required for cell separation during growth of Saccharomyces cerevisiae". The Journal of Biological Chemistry. 266 (29): 19758–19767. doi:10.1016/S0021-9258(18)55057-2. PMID 1918080.
- Penninga, D.; Van Der Veen, B. A.; Knegtel, R. M.; Van Hijum, S. A.; Rozeboom, H. J.; Kalk, K. H.; Dijkstra, B. W.; Dijkhuizen, L. (1996). "The raw starch binding domain of cyclodextrin glycosyltransferase from Bacillus circulans strain 251". The Journal of Biological Chemistry. 271 (51): 32777–32784. doi:10.1074/jbc.271.51.32777. PMID 8955113.
- Oyama, T.; Kusunoki, M.; Kishimoto, Y.; Takasaki, Y.; Nitta, Y. (1999). "Crystal structure of beta-amylase from Bacillus cereus var. Mycoides at 2.2 a resolution". Journal of Biochemistry. 125 (6): 1120–1130. doi:10.1093/oxfordjournals.jbchem.a022394. PMID 10348915.
- Armstrong, C. G.; Doherty, M. J.; Cohen, P. T. (1998). "Identification of the separate domains in the hepatic glycogen-targeting subunit of protein phosphatase 1 that interact with phosphorylase a, glycogen and protein phosphatase 1". The Biochemical Journal. 336 (3): 699–704. doi:10.1042/bj3360699. PMC 1219922. PMID 9841883.
- Boraston, A. B.; Healey, M.; Klassen, J.; Ficko-Blean, E.; Lammerts Van Bueren, A.; Law, V. (2005). "A Structural and Functional Analysis of -Glucan Recognition by Family 25 and 26 Carbohydrate-binding Modules Reveals a Conserved Mode of Starch Recognition". Journal of Biological Chemistry. 281 (1): 587–598. doi:10.1074/jbc.M509958200. PMID 16230347.
- Boraston AB, Revett TJ, Boraston CM, Nurizzo D, Davies GJ (June 2003). "Structural and thermodynamic dissection of specific mannan recognition by a carbohydrate binding module, TmCBM27". Structure. 11 (6): 665–75. doi:10.1016/S0969-2126(03)00100-X. PMID 12791255.
- Abbot, DW; Eirin-Lopez, JM; Boraston, AB (January 2008). "Insight into ligand diversity and novel biological roles for family 32 carbohydrate-binding modules". Molecular Biology and Evolution. 25 (1): 155–67. doi:10.1093/molbev/msm243. PMID 18032406.
- Ficko-Blean, Elizabeth; Boraston, Alisdair ,"Carbohydrate Binding Module Family 32" Archived 2016-08-20 at the Wayback Machine,CAZypedia, 4 May 2017.
- Schnellmann, J.; Zeltins, A.; Blaak, H.; Schrempf, H. (1994). "The novel lectin-like protein CHB1 is encoded by a chitin-inducible Streptomyces olivaceoviridis gene and binds specifically to crystalline alpha-chitin of fungi and other organisms". Molecular Microbiology. 13 (5): 807–819. doi:10.1111/j.1365-2958.1994.tb00473.x. PMID 7815940. S2CID 22470447.
- Vaaje-Kolstad, G.; Houston, D. R.; Riemen, A. H.; Eijsink, V. G.; Van Aalten, D. M. (2005). "Crystal Structure and Binding Properties of the Serratia marcescens Chitin-binding Protein CBP21". Journal of Biological Chemistry. 280 (12): 11313–11319. doi:10.1074/jbc.M407175200. PMID 15590674.
- Katsuya, Y.; Mezaki, Y.; Kubota, M.; Matsuura, Y. (1998). "Three-dimensional structure of Pseudomonas isoamylase at 2.2 Å resolution1". Journal of Molecular Biology. 281 (5): 885–897. doi:10.1006/jmbi.1998.1992. PMID 9719642.
- Wiatrowski, H. A.; Van Denderen, B. J.; Berkey, C. D.; Kemp, B. E.; Stapleton, D.; Carlson, M. (2004). "Mutations in the gal83 glycogen-binding domain activate the snf1/gal83 kinase pathway by a glycogen-independent mechanism". Molecular and Cellular Biology. 24 (1): 352–361. doi:10.1128/mcb.24.1.352-361.2004. PMC 303368. PMID 14673168.
- Polekhina, G.; Gupta, A.; Michell, B. J.; Van Denderen, B.; Murthy, S.; Feil, S. C.; Jennings, I. G.; Campbell, D. J.; Witters, L. A.; Parker, M. W.; Kemp, B. E.; Stapleton, D. (2003). "AMPK beta subunit targets metabolic stress sensing to glycogen". Current Biology. 13 (10): 867–871. doi:10.1016/S0960-9822(03)00292-6. PMID 12747837. S2CID 16778615.
- Hudson, E. R.; Pan, D. A.; James, J.; Lucocq, J. M.; Hawley, S. A.; Green, K. A.; Baba, O.; Terashima, T.; Hardie, D. G. (2003). "A novel domain in AMP-activated protein kinase causes glycogen storage bodies similar to those seen in hereditary cardiac arrhythmias". Current Biology. 13 (10): 861–866. doi:10.1016/S0960-9822(03)00249-5. PMID 12747836. S2CID 2295263.
- Urbanowicz BR, Catala C, Irwin D, Wilson DB, Ripoll DR, Rose JK (April 2007). "A tomato endo-beta-1,4-glucanase, SlCel9C1, represents a distinct subclass with a new family of carbohydrate binding modules (CBM49)". J. Biol. Chem. 282 (16): 12066–74. doi:10.1074/jbc.M607925200. PMID 17322304.